Alopecia areata is a T-cell mediated autoimmune disease of nonscarring hair loss with no effective treatment that induces and sustains remission. Apremilast, a small molecule inhibitor of PDE4 approved for the treatment of psoriasis and psoriatic arthritis, showed rapid and extensive scalp-hair regrowth in a patient who had psoriasis and ophiasic alopecia areata.
The association between alopecia areata (AA) and psoriasis is a well-known but rare condition [1]. AA management in daily clinical practice remains unsatisfactory. We report herein, for the first time, a patient who had remarkably extensive scalp-hair growth while she had been receiving Apremilast for psoriasis localized in the scalp and body.
A 57-year-old woman with a seven-year history of psoriasis and psoriatic arthritis was referred to our department, because at the time of psoriasis, she had also developed ophiasic alopecia areata. Psoriasis was treated with topical steroids, vitamin D analogues, UVB narrow band, and methotrexate, but they were ineffective. Treatment for alopecia areata included topical clobetasol propionate and minoxidil 5%, but there was no remarkable hair growth.
Dermatological examination revealed typical psoriasis plaques in both alopecic and non-alopecic areas of the scalp, arms, and trunk with initial Psoriasis Area and Severity Index (PASI) of 12, Body Surface Area (BSA) of 16, Physician's Global Assessment (PGA) of 3, and Scalp PGA of 4. It was remarkable that the patient complained of very severe itching (Itch Numeric Rating Scale of 8) and extensive hair loss of the temporal, parietal, and occipital regions of the scalp (Fig. 1).
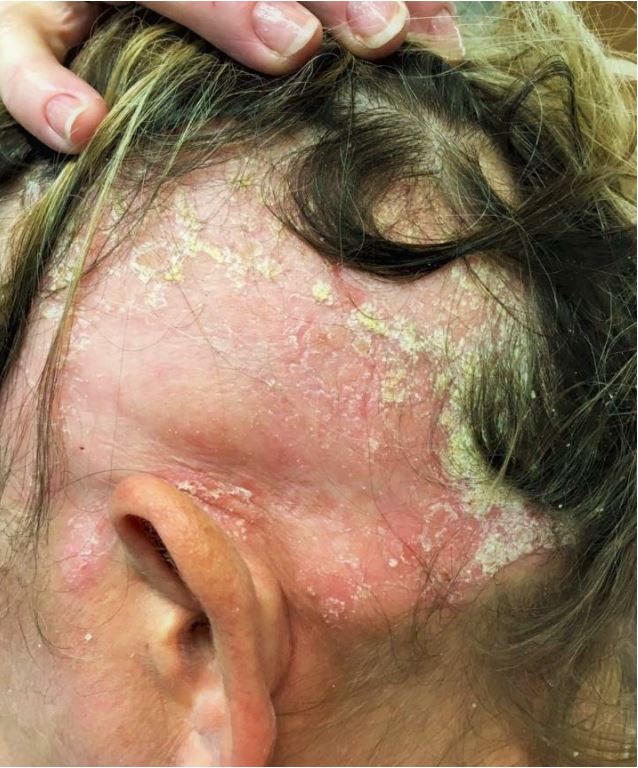
Figure 1. Ophiasic alopecia areata before treatment with Apremilast.
There was no family history of AA or psoriasis. Rheumatologist consultation and radiography confirmed the diagnosis of psoriatic arthritis. Laboratory findings showed no abnormalities.
Treatment with Apremilast was started as label dosing, and after 6 weeks the patient showed significant improvement in psoriasis lesions with reduced erythema and scaling, and marked improvement of pruritus. Interestingly, significant hair regrowth was seen on the scalp (Fig. 2), and with the ongoing Apremilast therapy, hair continued to grow until it achieved complete regrowth.
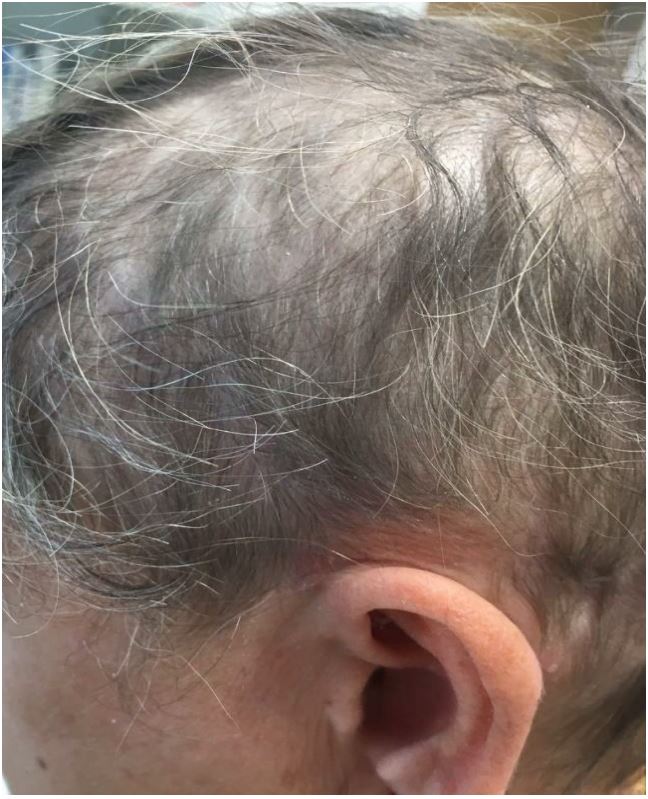
Figure 2. Significant scalp-hair regrowth after 6 weeks with Apremilast.
The patient developed diarrhea attributed to Apremilast since the first week, and subsequently weight loss (seven kilograms in 8 weeks). As the patient declined to stop the treatment, the drug use was continued, associating it with an oral antidiarrheal agent as loperamide and probiotics.
After twelve weeks, there was a successful hair regrowth on the scalp, in particular, in the areas with psoriasis lesions. The patient`s scalp hair was approximately 5 cm long with no remaining patches of AA (Fig. 3). Alopecia areata and psoriasis scalp were completely resolved. As the gastrointestinal side effects remained despite the complementary treatment, the patient agreed to stop the treatment. Six months later, the patient is still asymptomatic.
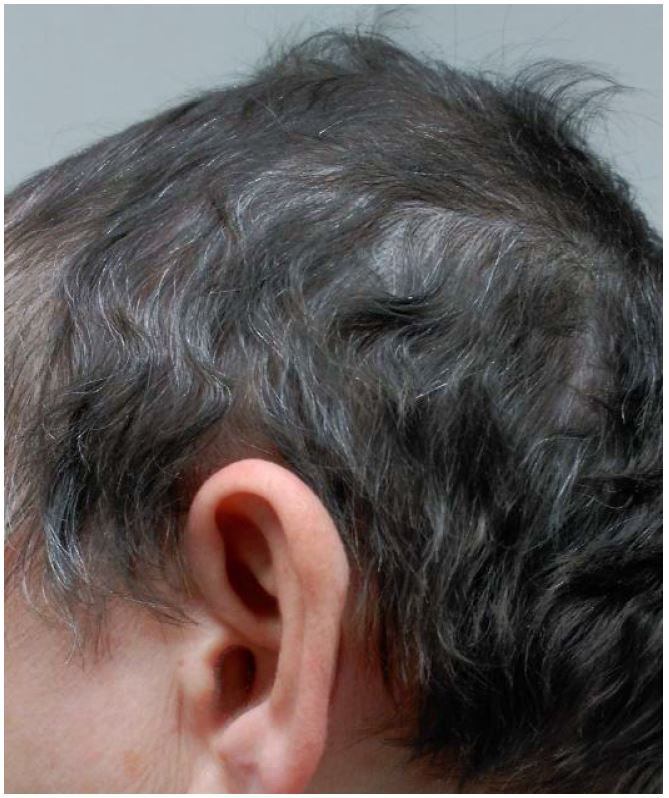
Figure 3. Total scalp-hair regrowth with a cosmetically important response after 12 weeks with Apremilast.
While many factors have been implicated in the pathogenesis of AA, it is now clear that the immune system is the major player, with T cells and a collapse of the physiological immune privilege of the hair follicle playing critical roles [2]. Although the Th1 pathway has been suggested as pivotal in the disease, recent studies suggest that Th2, Th9, phosphodiesterase (PDE) 4, and IL-23 axes might contribute to AA pathogenesis [3]. Animal models have greatly helped to elucidate critical cellular and molecular immune pathways in AA. Instead, a humanized mouse model of AA has been used to demonstrate the previously hypothesized key role of CD8+ T cells and NKG2D+ cells in AA pathogenesis [4].
PDE4 inhibitors (Apremilast) showed very recently the efficacy in treating AA in a humanized AA mouse model that employs normal human scalp skin and PBMCs from healthy donors that are transplanted onto SCID/Beige mice [5]. In that paper, the group of mice treated with Apremilast showed almost complete absence of CD8+, NKG2D+ cells and reduced production of pro-inflammatory cytokines, such as IFN-gamma and TNF-alpha. PDE4 was also highly increased in human AA lesions.
Psoriatic alopecia is difficult to distinguish from AA [6]. The frequency of AA, the ophiasic pattern of the alopecia in our patient, and the presence of yellow dots in the dermoscopy led us to consider the diagnosis of AA as more probable. The gastrointestinal side effects were also remarkable in our patient. Although the weight loss was thought to be independent of diarrhea, and usually it was found to be 1.5 kg on average over a one-year period, we thought this was not the case in our patient, and we finally decided to stop the treatment [7, 8].
In this case report, it was interesting to observe the amazing effectiveness of Apremilast in refractory ophiasis AA with a rapid and highly scalp-hair regrowth with a cosmetically important response.
Received date: July 07, 2017
Accepted date: August 28, 2017
Published date: September 27, 2017
© 2017 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Based on the observation, if the author believes in the finding and the rationale, the author should recommend further studies.
ResponseModifications have been done as per the suggestions; thanks.
The revised article can be accepted for publication.
The article is accepted.