Background: Decontamination agents of flexible nasoendoscopes (FNE) are efficacious in-vitro. However, few studies evaluated their efficacy in the clinical setting.
Objectives: To evaluate the clinical efficacy of Anioxyde 1000 (5-minute clinic process) and automated-washer (30-minute centralized process) in FNE decontamination, and to examine the bio-burden of the FNE following its use.
Study Design: Prospective study.
Results: Microbiological swabs were taken sequentially before (swab 1/storage practice) and after (swab 2/positive control) FNE usage, and also after decontamination with Anioxyde 1000 (swab 3/ Anioxyde 1000 efficacy) as well as automated-washer (swab 4/automated-washer efficacy); the bacterial growth was reported in 6/60, 57/60, 6/60, and 4/60 swabs respectively. Both Anioxyde 1000 and automated Washer are efficacious in decontamination in relation to positive control (p=0.000), with no statistically-significant difference between the efficacy of Anioxyde 1000 and automated-washer (p = 0.727). Coagulase Negative Staphylococci, Staphylococcus aureus, and Staphylococcus epidermidis were the commonest isolates on FNE after use, with no spore-forming or strict pathogens isolated.
Conclusion: Clinical decontamination of FNE with Anioxyde 1000 is effective and resource-efficient, which supports the use of this decontamination process in clinics.
Flexible nasoendoscope decontamination has become a subject of immense importance and relevance, especially with regard to patient safety. To illustrate, over the last decade, flexible nasoendoscope has become an indispensable tool to otorhinolaryngologists. It has become part of the routine clinical examination, providing instant and clear visualization of the nasal cavity, pharynx, and larynx in the clinic setting. The average clinic uses the flexible nasoendoscope about 7 times in one session [1]. After each use, the flexible nasoendoscope needs to be decontaminated prior to its use on the next patient in order to avoid the risk of cross- infection among patients.
In recent years, contention has been raised regarding the need for centralization of the decontamination process [1,2]. Current guidance advocates that the automated washer be operated in a centralized decontamination unit [3]. The rationale is that the automated washer has the advantage of being operated on a preset protocol, which is repeatable and can be regularly tested. The centralization of decontamination
process also ensures that trained staff would be handling the decontamination process on a high-volume basis. Overall, this minimizes variability and potentiality for errors in the decontamination process. However, centralization of the decontamination process comes with its own set of problems, which include prolonged decontamination time necessitating additional purchase of scopes to cope with clinical turnover, additional costs and manpower for transport of scopes to a centralized unit [1].
Most of the guidelines on flexible nasoendoscope decontamination are extrapolated from decontamination evidence of gastroscopes [1]. However, this application of evidence is flawed owing to the differences in nature of the procedures as well as the degree of contamination both types of scopes are exposed to. For instance, unlike gastroscopes, the most widely used flexible nasoendoscopes are those without biopsy channels. Secondly, unlike gastroscopy, flexible nasoendoscopy is also less liable to blood contamination. Hence, one may be able to argue that flexible nasoendoscopes without biopsy channels may not have to undergo a decontamination process as rigorous as that of gastroscopes, but still retain the clinical efficacy in preventing cross-contamination among patients, and at a cheaper cost.
Anioxyde 1000 is the regular, 5-minute decontamination process currently being used in our department. Although it has been proven effective as a decontaminating agent in laboratory studies, its efficacy in decontamination in the clinical setting has yet to be determined. It is important to prove efficacy in clinical use because the effectiveness of such decontamination agents is subjected to variables such as clinical demands for equipment turnover, the ease of use of the decontamination process, the effort being put into staff training, and the audit of clinical practices to ensure compliance with protocol. In this study, we will examine the efficacy and adequacy of our clinical decontamination practice.
The primary aim of our study is to examine the efficacy of flexible nasoendoscope decontamination utilizing the Anioxyde 1000 system and the automated washer in the clinical setting.
The secondary outcome measure is to examine the bio-burden of the flexible nasoendoscope following a scope procedure being done on patients. This will give us an idea of pathogenicity of the organisms and postulate the disease transmissibility of flexible nasoendoscopes.
This study received ethics approval from the Domain Specific Review Boards of National Healthcare Group, Singapore.
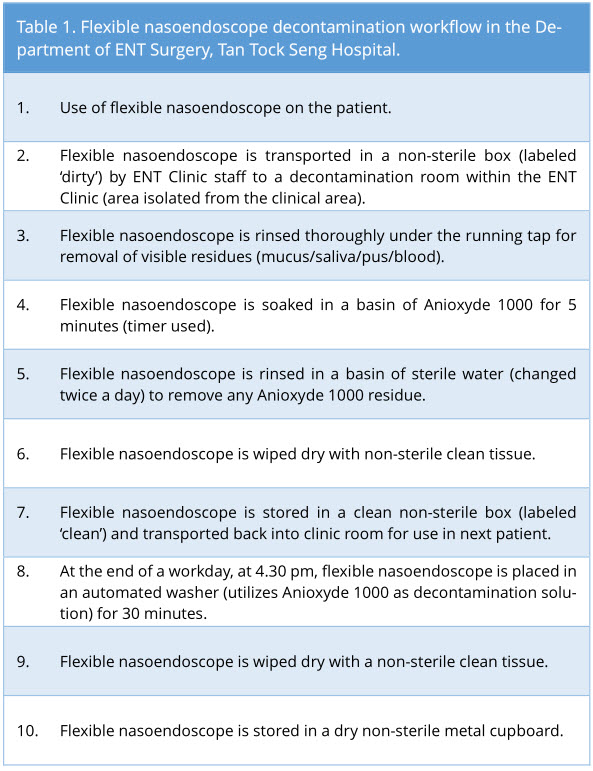
After routine use of the flexible nasoendoscope (Pentax® and Olympus®) in adult patients in our General and Subspecialty Otolaryngology clinics, flexible nasoendoscopes were decontaminated as per our standard clinic protocol (Table 1). Main decontamination process utilized is the soaking of flexible nasoendoscope in Anioxyde 1000. In accordance with the hospital guidelines, the flexible nasoendoscope is also decontaminated in an automated washer at the end of a workday.
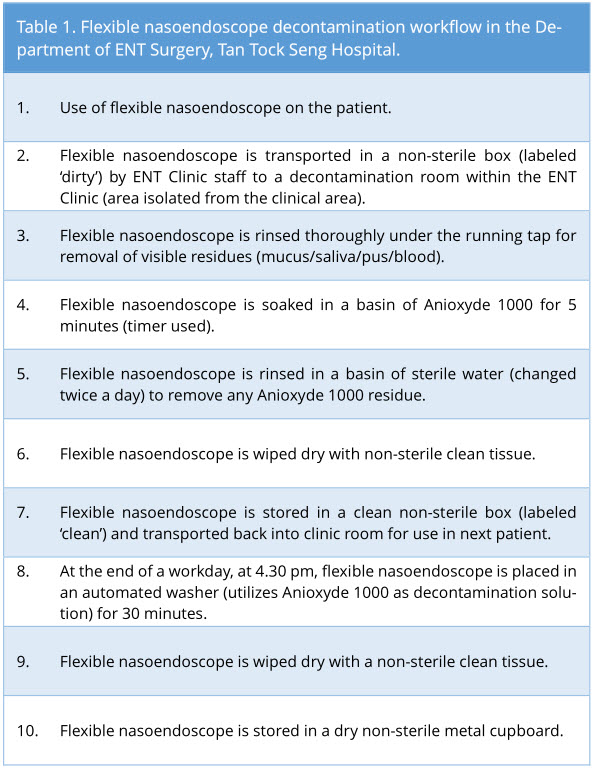
Sterile microbiological cotton swabs were used to swab the flexible nasoendoscope tips and surface area up to 2 cm proximal to the tip, with numerous passes back and forth within this area, to ensure the maximal yield of each swab. This is the area of the scope that is in most consistent contact with the patient.
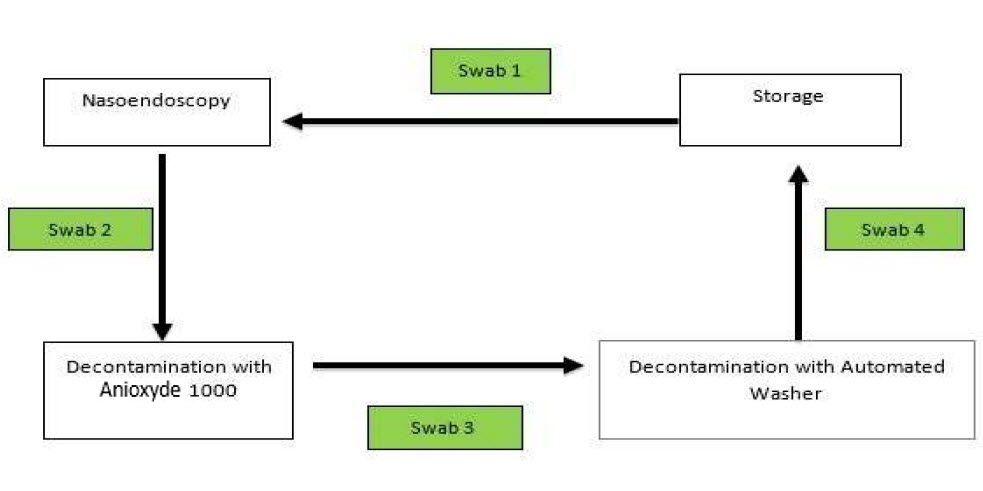
Swabs were taken sequentially in the decontamination workflow. Swab 1 was taken before flexible nasoendoscopy was performed. Swab 2 was taken after the flexible nasoendoscopy was performed on the patient (as shown in Figure 1). The subsequent swab 3 was taken after the flexible nasoendoscope was decontaminated with Anioxyde 1000.
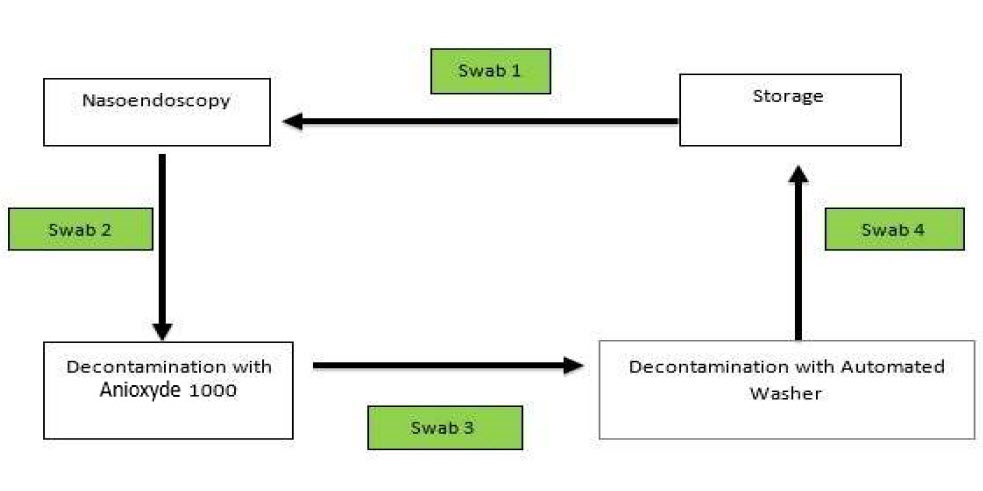
Figure 1. Sequence of which microbiological swabs were taken.
Specifically for this trial (deviation from clinic workflow), the flexible nasoendoscopes were then further decontaminated with an automated washer to prevent any risk of cross-infection in our patients from potential inadequate Anioxyde 1000 decontamination. Swab 4 was taken after the flexible nasoendoscope was decontaminated with the automated washer.
The rationale for each swab is as follows:
Anioxyde 1000
Anioxyde 1000 is a high-level decontamination agent that contains extemporaneous production of peracetic acid from acetylcaprolactam (PHERA system) and 3% hydrogen peroxide. It is bactericidal, fungicidal, virucidal and sporicidal within a contact time of 5 minutes [6].
Microbiological Swab Processing Protocol
After sampling, the swab was inoculated into 10 ml of brain-heart infusion broth (BHIB) and vortexed for 30 seconds. It was then incubated at 35°C for 7 days. The broth was observed for turbidity every other day up to Day 7. The broth was sub-cultured onto of blood agar (BA) and incubated at 35°C ambient air, if turbidity was noted. Organisms grown were identified using the Bruker MALDI Biotyper mass spectrometer (MALDI-TOFMS). If broth was clear by Day 7, it was reported as no growth.
McNemar test was used to analyze the difference in bacterial growth between swab 2 and 3, as well as swab 2 and 4, to assess the decontamination efficacy of Anioxyde 1000 and automated washer. It was also used to analyze the difference between swab 3 and 4 to allow comparison between Anioxyde 1000 and automated washer.
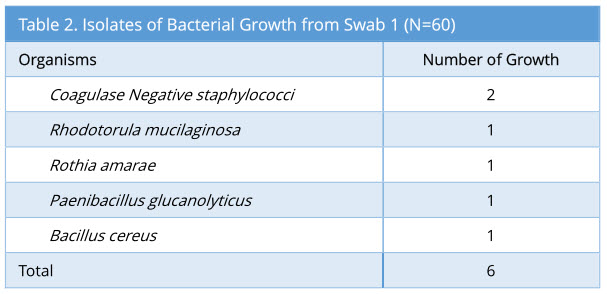
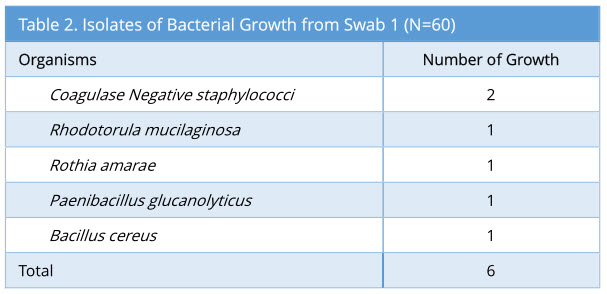
A total of 60 sets of swabs were taken sequentially as the decontamination process was carried out. Swab 1 showed 6/60 growth (10%). The profile of bacterial growth isolated is as below (Table 2).
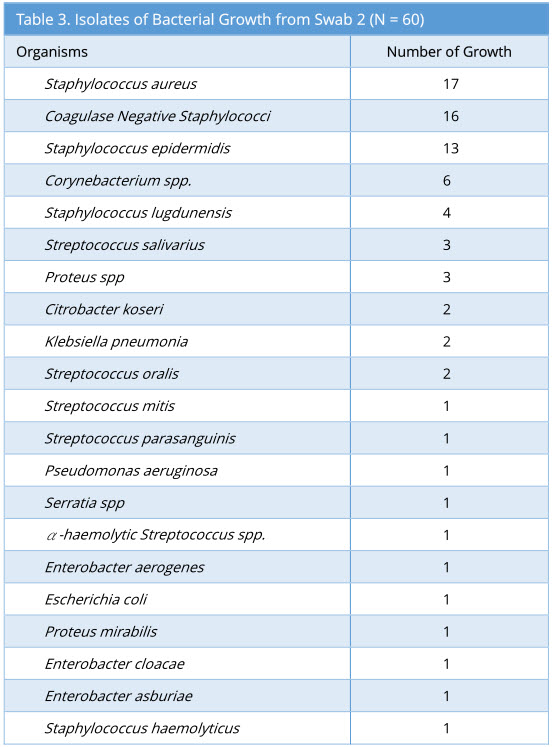
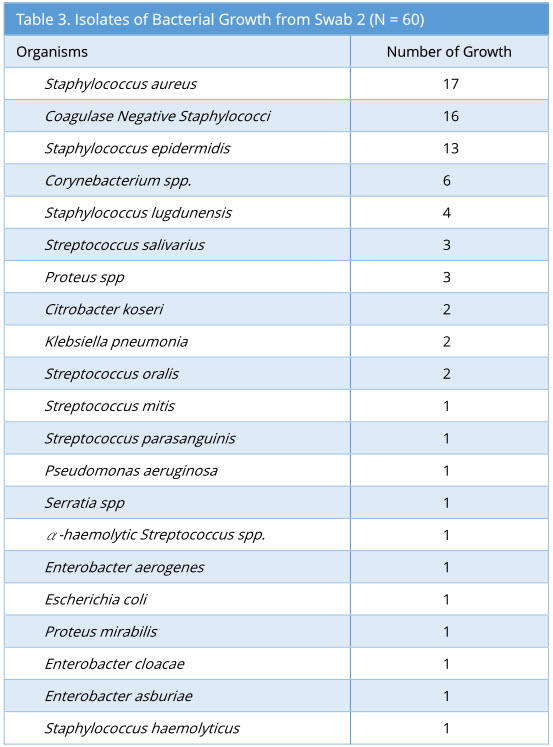
Swab 2 showed 57/60 growth (95%). Some swabs showed more than one type of bacterial isolate. The profile of bacterial growth isolated is as below (Table 3).
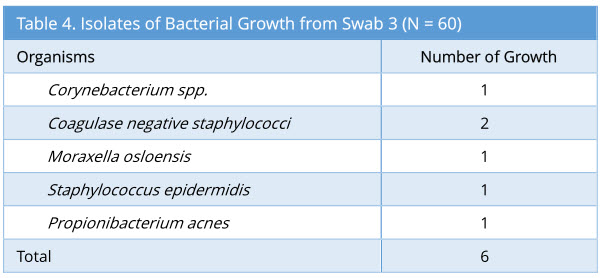
Swab 3 showed 6/60 growth (10%). The profile of bacterial growth isolated is as below (Table 4). Out of the 6 growth isolated, none of these were the same organism isolated from the corresponding swab 2.
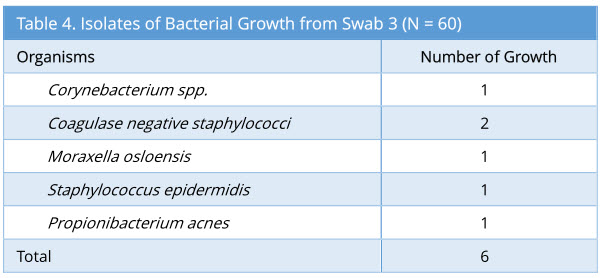
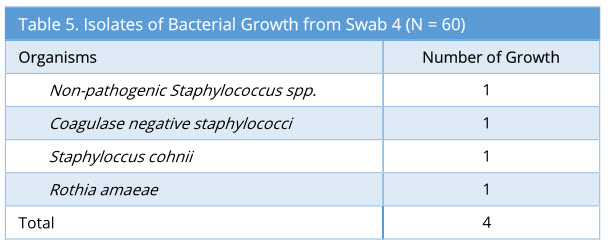
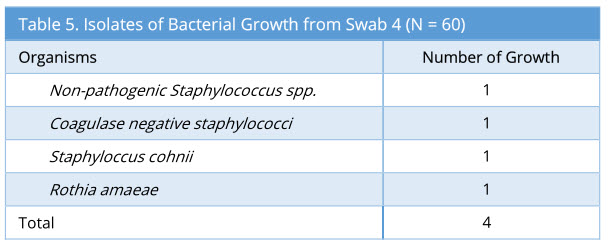
Swab 4 showed 4/60 growth (7%). Out of the 4 growth isolated, none of these were the same organism isolated from the corresponding swab 2 and swab 3. The profile of bacteria growth isolated is as below (Table 5).
Anioxyde 1000 (swab 3) is efficacious in decontamination compared to that of the positive control (swab 2) (p=0.000 McNemar's Test). Similarly, automated washer (swab 4) is efficacious in eliminating bacterial growth when compared to positive control (swab 2) (p=0.000 McNemar’s Test). There was no statistical significance in the decontamination efficacy of Anioxyde 1000 (swab 3) and automated washer (swab 4) (p = 0.727 McNemar’s Test).
There was no damage to nasoendoscopes sustained as a result of the decontamination process during the study period.
The regular use and rapid turnover of flexible nasoendoscopes in the clinic setting pose a potential risk of cross infection. This is because an inadequately decontaminated flexible nasoendoscope serves as a potential avenue for disease transmission from one patient to another. Our study aims to investigate this element of healthcare.
It is most important to note that the flexible nasoendoscope decontamination process is a non-sterile process. From the storage, handling, and clinical use, to the decontamination of the scopes, for practical reasons, it is not done in an aseptic manner. Traditionally, there has not been any requirement for flexible nasoendoscopes to be stored in a sterile environment. It is advocated that it should be stored by hanging it vertically in a dry clean cupboard [4]. However, the safety of such practice was not previously investigated. Our study demonstrated minimal non-pathogenic environmental growth on our flexible nasoendoscope with non-sterile storage. This demonstrates that it is both practical and adequate for flexible nasoendoscopes to be stored in a non-sterile but clean and dry environment.
Swab 2 is taken after the flexible nasoendoscopy is performed on the patient and before any decontamination is done. In this study, it functions as a positive control. In addition, swab 2 also reflects the bio-burden on the flexible nasoendoscopes following routine flexible nasoendoscopy. In our study, Coagulase Negative Staphylococci, Staphylococcus aureus, and Staphylococcus epidermidis were the commonest isolates on flexible nasoendoscopes following the process of flexible nasoendoscopy on our ENT patients. These are similar to the organisms found in the normal flora of upper respiratory tract as shown by Jousimies-Somer et al [5].
Our study also showed that there were no spore-forming bacteria or strict pathogens (pathogens that can infect all human hosts who are exposed to it such as Mycobacterium tuberculosis, Treponema pallidum, Plasmodium, Neisseria gonorrhoeae) isolated following routine flexible nasoendoscopy. This finding has clinical implications in rationalizing the use of high-level disinfection in flexible nasoendoscope decontamination. Controversies exist with regard to whether healthcare instruments should be sterilized or high-level disinfected. Although high-level disinfection is deemed as the standard and adequate decontamination process utilized for flexible nasoendoscopes [6], it has the disadvantage of not being able to eliminate bacterial spores. Yet, many scopes used in the clinical setting, including the flexible nasoendoscopes, are heat sensitive and cannot undergo sterilization. In our study, the low pathogenicity of organisms and absence of spore-forming bacteria isolated from our flexible nasoendoscopes following use in patients suggest that high-level disinfection is adequate. This is further supported by the absence of reports of cross-infection from flexible nasoendoscope use.
Unlike some of the other recent studies [7-9], some of our swabs (swab 3 and 4) showed some bacterial growths following decontamination with Anioxyde 1000 and automated washer. However, none of these growths are bacterial isolates from the corresponding swab 2. This suggests that the decontamination process was indeed effective in removing the bacteria following flexible nasoendoscopy. However, the different bacterial growth isolated from swab 3 and swab 4 suggests environmental origins of these contaminants. Given that the decontamination process is not done entirely in a sterile manner, it is plausible that the contaminants could originate from non-sterile gloves or from airborne contaminants (staff sneezing/coughing).
In recent years, there has been an increased movement towards the use of automated washer in a centralized decontamination unit in order to reduce processing error from human variability. However, the recently published studies showed that simple and quicker decontamination processes were as efficacious in decontamination compared with the automated washer [7,8]. Liming et al demonstrated that ‘30-second 70% isopropanol + antimicrobial soap scrub’ or a ‘12-minute Cidex OPA soak’ is just as effective as the ‘30-minute run in an automated endoscope reprocessor’ [8]. Our results demonstrated that the 5-minute Anioxyde 1000 soak used in the clinic and a non-centralized decontamination setting are effective in bacterial elimination. The emergence of such clinical evidence demonstrating equal efficacy of these shorter and simpler decontamination protocols has potential implication in driving the decontamination process towards less time-consuming methods. This could help alleviate the manpower burden required to transport the scopes to the automated washer. At the same time, it obviates the necessity to purchase more flexible nasoendoscopes to cope with the prolonged automated washing process and rapid clinical turnover.
One of the limitations of our study is the lack of investigation into fungal and virus growth in the decontamination process. This would have provided a more complete picture of the bio-burden on the flexible nasoendoscope following the procedure and thus presented a clearer picture on the disease transmissibility of a routine flexible naso-endoscopy procedure. This limitation is due to cost constraints. However, it is important to note that Peracetic acid has been shown to inactivate fungi, yeast, and virus and eliminate spores effectively in the in vivo setting [10]. In addition, a previous clinical study has demonstrated low fungal contamination from the flexible nasoendoscope following the scope procedure [7].
Another area that could be evolved from our study is that the microbiological swabs can be taken from consecutive patients. As a proposed study for future, testing of decontamination efficacy in sequential patients will be helpful to evaluate the disease transmissibility of flexible nasoendoscopes following inadequate decontamination.
Our existing clinical decontamination process with a 5-minute soak of Anioxyde 1000 is effective. It is adequate for flexible nasoendoscopes to be stored in a non-sterile but clean and dry environment. Bio-burden testing of flexible nasoendoscope showed similar growth to that of the organisms found in the normal flora of upper respiratory tract. However, virus, fungi and sporicidal growth were not investigated in our study. Hence, the full spectrum of bio-burden of flexible nasoendoscopy requires further examination.
Received date: August 06, 2017
Accepted date: September 07, 2017
Published date: September 26, 2017
© 2017 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Decontamination of scopes is a very important subject. Deaths are reported using various scopes not properly decontaminated. There are significant lawsuits against decontamination agents that fail. Because of this history, few readers would venture to switch to a different method, unless the significant detail is demonstrated thoroughly. This is not a light subject. The more you can make this a major article, the greater the interest in your subject.
The authors did two similar studies before using chlorine dioxide wipes and automated washing and obtained the same results (Sequential cohort study comparing chlorine dioxide wipes with automated washing for decontamination of flexible nasoendoscopes. J Laryngol Otol. 2012, 126(8):809-14. Audit of flexible nasoendoscope decontamination – Clinical efficacy and cost effectiveness. International Journal of Surgery. 2011, 9(7): 582).
In this manuscript, the authors changed chlorine dioxide wipes into Anioxyde 1000 soak. The results indicated that samples from chlorine dioxide wipes showed 2% (1 out of 50 swabs) growth of Staphylococcus epidermidis. It seems like chlorine dioxide wipes are more effective than Anioxyde 1000 soak. So, the decontamination with chlorine dioxide has more significance in practice. The authors should explain it in the DISCUSSION to strengthen the rationale of the present manuscript and express their innovative ideas.
ResponseAs the study did not compare ANIOXYDE 1000 with chlorine dioxide (and therefore no statistical tests can be done to compare between the two), we are unable to comment on whether chlorine dioxide is superior, equivalent, or inferior to ANIOXYDE 1000. This may perhaps be useful in future studies to determine the decontamination agent of choice.
In regards to Swabs 3 and 4, are they from the same nasoendoscope? What are the total sample size and the sample size for each group?
ResponseYes, swabs 3 and 4 are from the same nasoendoscope. The total sample size is 60 (with a total of 60 x 4 = 240 swabs taken, as 4 swabs are taken at each step per endoscope).
I have checked the revised article. It can be accepted for publication.
The article is good, please publish it.