Flow-through flaps, wherein two or more flaps are placed in series using microvascular anastomoses, are indicated for the coverage of longer and wider defects, typically in extremities. These flaps are technically demanding but, more importantly, are associated with distal flap loss. To overcome these problems, the ‘Orochi’ concept of placing multiple flaps in parallel as part of a modular reconstruction has been put forward. In this article, this concept is illustrated in two cases of extremity reconstruction with the use of a sequential triple thoracodorsal perforator (TAP)-intercostal artery perforator (ICAP)-superficial circumflex iliac artery perforator (SCIP) mega-flap placed in parallel with a free TAP flap at the distal end of the defect. These modular mega-flaps are connected to a common vascular trunk by means of end-to-side microvascular anastomoses or perforator-to-perforator anastomoses to create parallel vascular systems; hence, the ‘Orochi’ (hydra-like) analogy. This allows for extensively long extremity defects of over 50 cm to be reconstructed safely. The use of the TAP-ICAP-SCIP model also confers the advantage of minimising operating time, negating the need for repeated positional change as with multiple flap elevation and a relatively concealed donor site.
The reconstruction of longer and wider defects in extremities remains a challenge in the reconstructive setting. The extended latissimus dorsi musculocutaneous (LDMC) flap and anterolateral thigh (ALT) flap have longer vascular dimensions [1], but are still disadvantaged by the limited dimensions of a single angiosome and invasive donor sites. Hence, the need for lateral thinking to create a new flap or flap combinations with a longer reach alongside the reduced donor site morbidity. Sequential LDMC and groin flap or erstwhile named ‘siamese’ flaps had a great advantage for longer defects in the extremity. However, the major problems are (1) short pedicle length of the superficial circumflex iliac artery (SCIA) system, which restricts its longitudinal dimension, (2) positional change for flap elevation, and (3) requiring the sacrifice of the latissimus dorsi muscle.
To overcome the problems of sequential LDMC-groin flap without having to resort to flow-through flaps, we developed a combination of a free thoracodorsal artery perforator (TAP) and sequential TAP-intercostal artery perforator (ICAP)-superficial circumflex iliac artery perforator (SCIP) flaps. The latter used triple perforator flaps of the TAP, ICAP, and SCIP in sequence with additional supercharging. Unlike flow-through flaps, in which flaps are placed in series with a proportionate decrease in the hydrostatic pressure within the main vascular axis, the creation of parallel vascular pathways as shown in this article allows for the successful reconstruction of very long and narrow defects.
In a retrospective study of two cases of extremity reconstruction, the ‘Orochi’ flap concept is illustrated as a multi-staged procedure. In the first stage, a free TAP flap is raised with the patient in the supine position, with a width of less than 8 cm, allowing for direct closure. The TAP flap is then inset at the distal end of the defect with anend-to-side or perforator-to-perforator microvascular anastomosis, thereby creating a parallel vascular system. At the next stage, a sequential TAP-ICAP-SCIP triple mega-flap is raised using two concurrent surgical teams operating in the axillary and groin areas, respectively. Bloodless elevation is very important so as not to damage the perforators. For this purpose, the flap is elevated subfascially using cautery scissors, even around the perforators of the flap pedicle. The length of the SCIP flap’s vascular pedicle can be increased by further dissecting the SCIA [2], allowing for greater ease of pivoting the flap. This manouevre necessitates the division of the lateral femoral cutaneous nerve (LCFN) as the SCIA system passes beneath it. The LCFN is subsequently coapted again after flap elevation [2].
As this mega-flap transverses three angiosomes, viz. the SCIP, TAP, and ICAP systems, the ICAP in the middle third of this sequential flap should also be dissected akin to the TAP and SCIP pedicles, and transected near the rib. This maximises the pedicle length. Its inclusion allows for an additional option of supercharging the mid-third of the mega-flap in case it is poorly vascularized. This may be needed if the ICAP system has hypoplastic or absent vascular channels. Once the flap is raised, it is pivoted on the TAP or SCIP pedicle, depending on whether the defect to be reconstructed is on the upper or lower extremity. Once inset, the distal flap vascular pedicle is supercharged in parallel to the main vessel via end-to-side or perforator-to-perforator anastomoses to further vascularise the mega-flap.
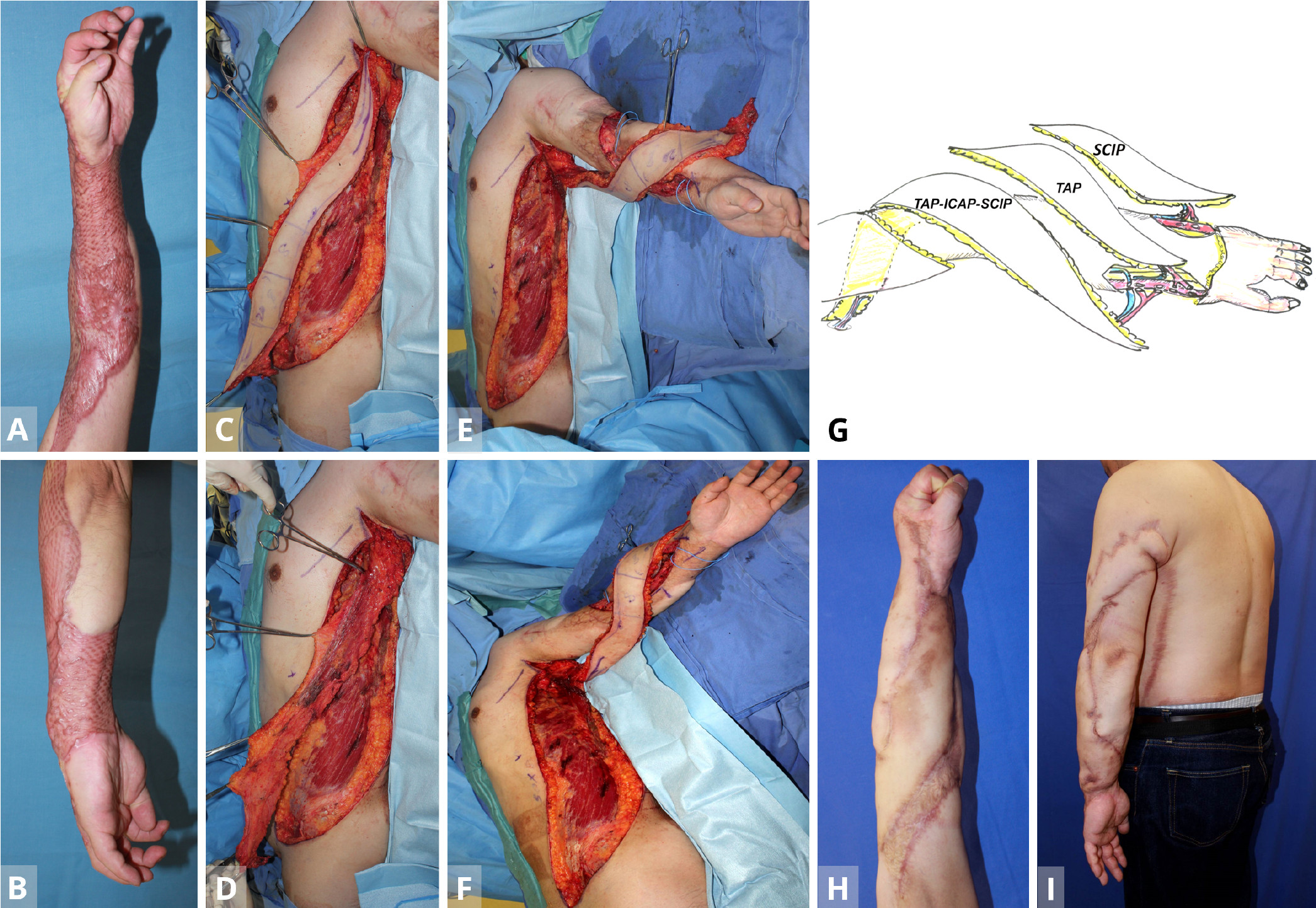
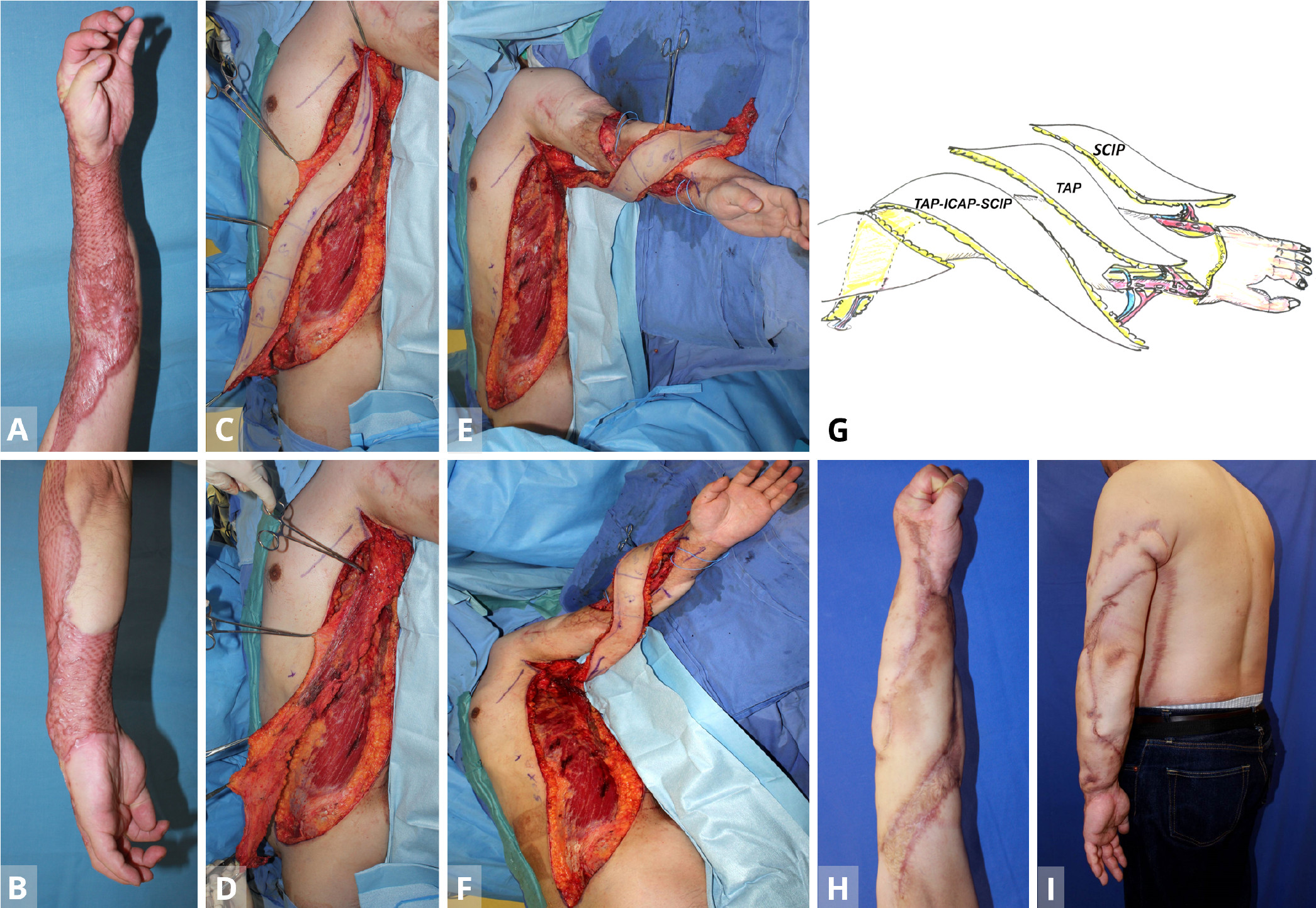
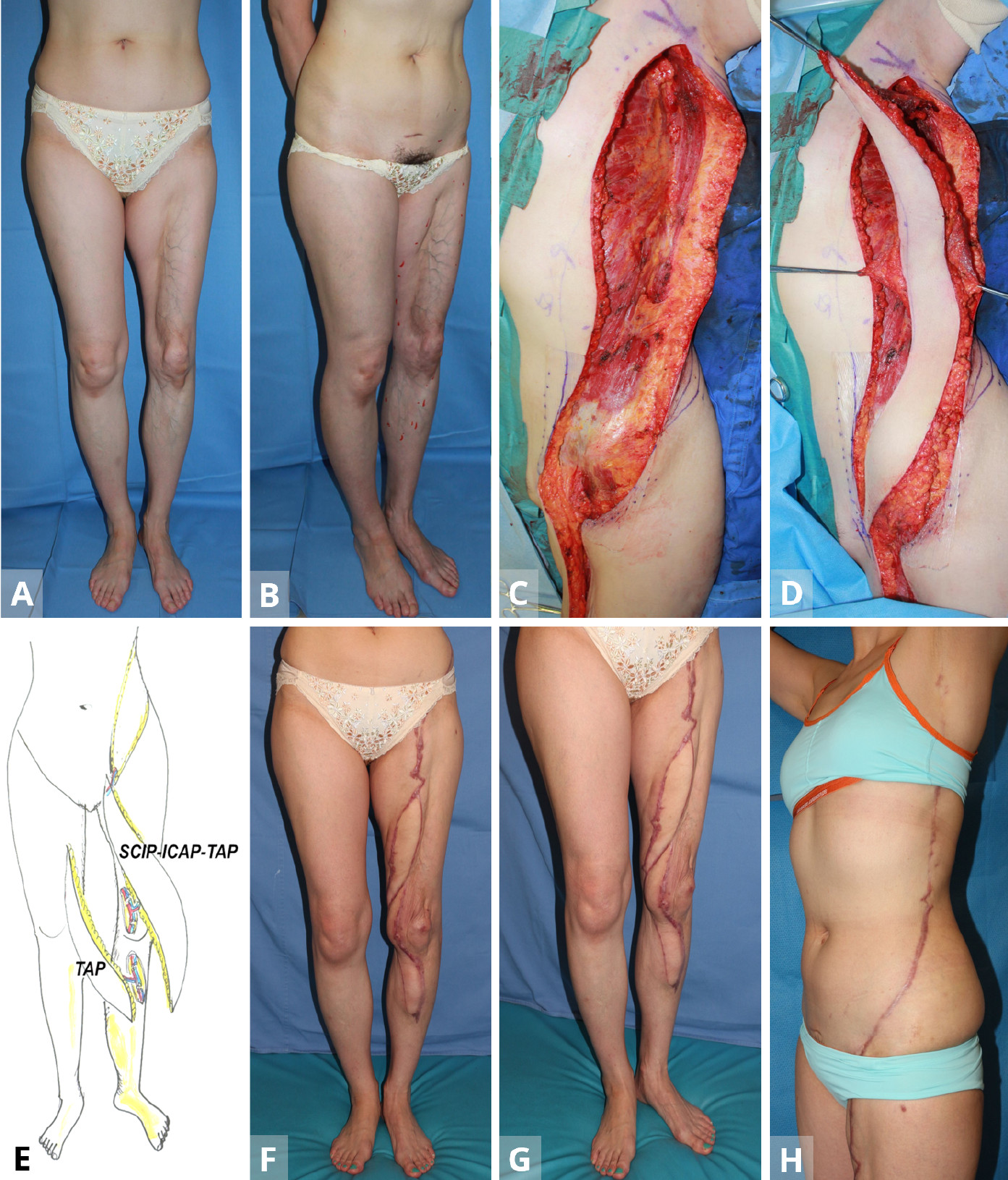
Figure 1. Case 1. (A,B) A 35-year old man with severe contracture due to mesh skin graft after wide necrotizing fascitis. The patient was unable to flex fingers. (C,D) After the primary free TAP flap is transferred from the contralateral lateral thoracic region for the dorsal forearm (stage 1), the TAP-ICAP-SCIP lap is elevated to reconstruct the volar forearm and upper arm defect (stage 2). (E,F) The triple TAP-ICAP-SCIP mega-flap in the second stage is raised and then inset to cover the more proximal spiral 50 x 8 cm defect after releasing the contracture. (G) In the first stage, a free TAP flap from the contralateral middle axillary region is transferred to the posterior aspect of the forearm. Using end-to-side arterial anastomosis, the T-shaped pedicle of the TAP flap is anastomosed with the recipient radial vessel at the wrist level, creating a parallel vascular system to sustain the TAP flap. At the second stage, a sequential 50 x 8 cm TAP-ICAP-SCIP mega-flap inset into the spiral defect from the proximal upper arm to the flexor side of the forearm. The SCIP component is distally supercharged by the recipient radial vessels at the level of the wrist (end-to-side) arterial anastomosis. At the third stage, contralateral SCIP flap is transferred to the ulnar aspect of the forearm. The superficial circumflex iliac artery and the cutaneous vein of SCIP flap are anastomosed to the recipient ulnar artery (end-to-side) and concomitant vein at the level of the wrist joint. (H,I) Normal power grip at five years later. The majority of the entire split skin graft is replaced with sequential triple TAP-ICAP-SCIP mega-flap, all in parallel. ICAP, intercostal artery perforator; SCIP, superficial circumflex iliac artery perforator; TAP, triple thoracodorsal perforator.
A 35-year-old carpenter developed necrotizing fascitis of a wide area of the left arm after a contaminated nail puncture injury. After radical debridement of soft tissue, the defect was initially reconstructed with a meshed split skin graft, which, however, caused severe scar contracture of the entire forearm and lateral portion of the upper arm, in the longer term (Figures 1A,B). In a subsequent surgery in the supine position, a free TAP flap (35 x 8 cm) from the contralateral middle axillary region was transferred to release the contracture of the posterior aspect of the forearm and hand. Using end-to-side arterial anastomoses, the T-shaped pedicle of the TAP flap was anastomosed with the recipient radial vessel at the wrist level, creating a parallel vascular system to sustain the TAP flap. Donor site was closed directly.
At the second stage on June 25, 2012, namely a year later, a sequential 50 x 8 cm TAP-ICAP-SCIP mega-flap from the ipsilateral left side was raised, with the patient again in the supine position (Figures 1C,D). The flap was pivoted on the TAP pedicle, with the distal SCIA system of SCIP flap being transected to allow the flap to be pivoted and inset into the spiral defect. This allowed coverage of the defect from the proximal upper arm to the flexor side of the forearm. The SCIP component of the triple mega-flap was distally supercharged between the SCIA (artery) and the cutaneous vein of SCIP flap (vein) and the recipient radial vessels at the level of the wrist (end-to-side arterial anastomosis (Figures 1E-G). The donor defect was again closed primarily.
At the third stage, the contralateral SCIP flap was transferred to release contracture of the ulnar aspect of the forearm. The SCIA and the cutaneous vein of SCIP flap were anastomosed to the recipient ulnar artery (end-to-side) and concomitant vein (end-to-end) at the level of wrist joint (Figure 1G). Subsequently, the narrow skin area of these flaps was widened using silicone expanders (Figures 1H,I).
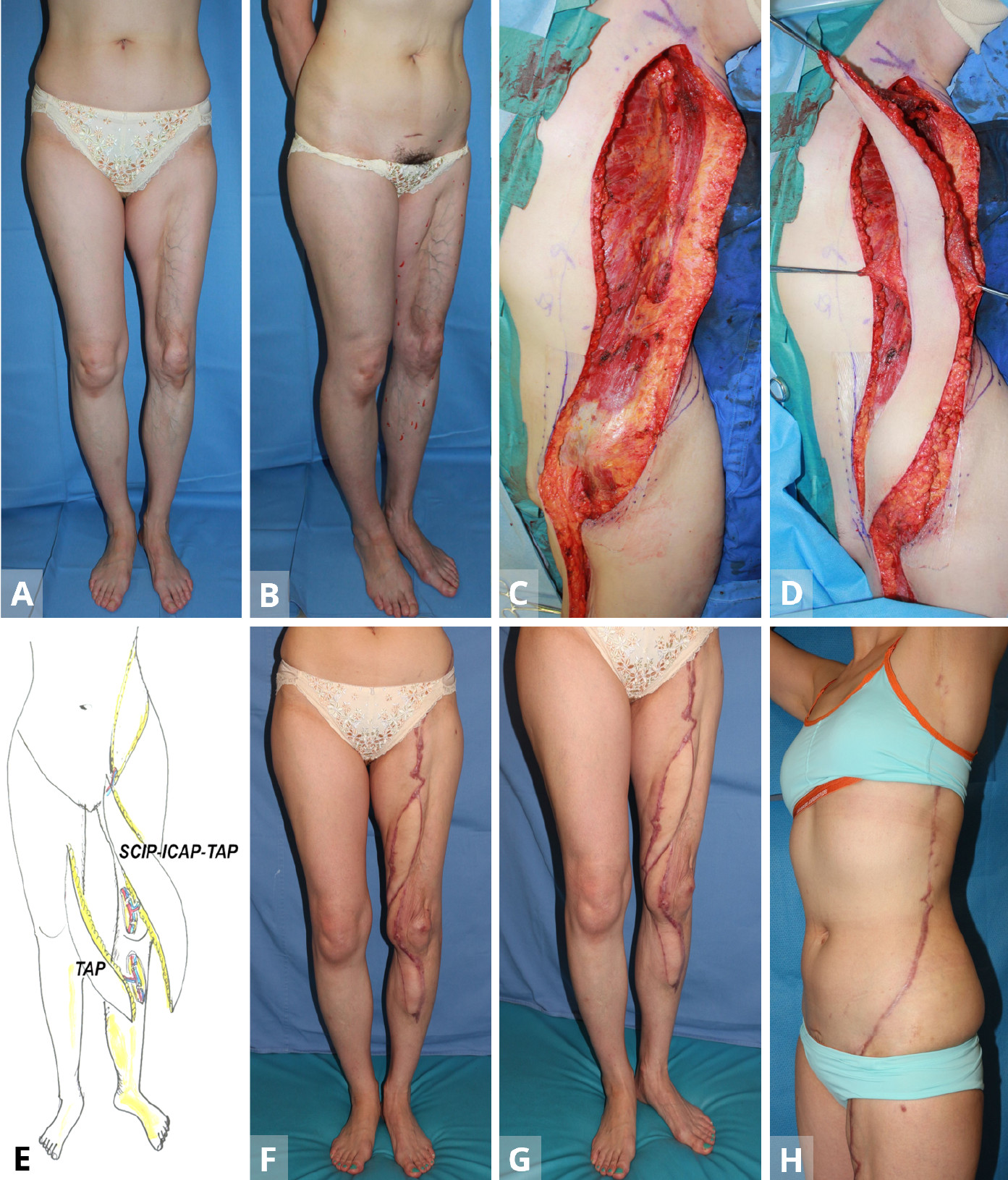
Figure 2. Case 2. (A,B) A 45-year-old woman with lipodystrophy on the anterior aspect on her left leg. (C,D) Following the primary TAP free-flap transfer from the contralateral side for lower leg, in the secondary surgery, the sequential TAP-ICAP-SCIP flap is elevated from the ipsilateral left side. (E) The first stage entails the use of the free TAP from the contralateral middle axillary region. The initial defect reconstructed is the distal-most part of the overall defect. During the secondary surgery, a sequential triple mega-flap mega flap (50 x 9 cm) based on the superficial circumflex iliac artery pedicle is pivoted into the longitudinal defect of the thigh. Supercharge of the distal pedicle is established between the transected TAP vessels and the perforators around the knee (recipient vessels) to create a parallel vascular system to the main vascular trunk of the lower limb. (F-H) Postoperative appearance at twenty months after completion of the ‘Orochi’ flap. ICAP, intercostal artery perforator; SCIP, superficial circumflex iliac artery perforator; TAP, triple thoracodorsal perforator.
A 45-year-old woman had lipodystrophy over the anterior aspect of her left thigh as well as the medial aspect of the left knee (Figures 2A,B). As in Case 1, the first stage entailed the use of the free TAP (30 x 7 cm), in the supine position from the contralateral middle axillary region. The initial defect reconstructed was the distal-most part of the overall defect. Microvascular anastomosis was then performed with an end-to-end, perforator-to-perforator anastomosis between the flap pedicle and recipient perforators around the knee. Donor site was closed directly.
During the secondary surgery on March 22, 2013, a sequential TAP-ICAP-SCIP flap (50 x 9 cm) was again elevated from the ipsilateral left side in a spine position (Figures 2C,D). To cover a lower limb defect, the flap was based on the SCIA pedicle and pivoted into the longitudinal defect, proximal to the free TAP flap (stage 1). Supercharge of the distal pedicle was then established between the transected TAP vessels and the perforators around the knee (recipient vessels) via an end-to-end microvascular anastomosis to create a parallel vascular system to the main vascular trunk of the lower limb (Figure 2E) . The donor site was closed primarily. Follow-up at twenty-months did not reveal any donor-site morbidity or functional disturbances (Figures 2F-H).
In both of these cases, the postoperative course was smooth with no evidence of partial or total flap necrosis. Follow-up at five-years (Case 1) and twenty-months (Case 2) documented improvements in both functional and aesthetic terms, both in the donor and recipient areas (Figures 1G, 2E).
The reconstruction of very long and narrow defects, specifically of the extremities, has previously been described with the use of flaps such as the LDMC-groin flap [1], bilateral deep inferior epigastric artery perforator (DIEP) flaps [3-5], and the turbo-charged gracilis myocutaneous flap [6]. Others have similarly reported the Tensor Fasciae Latae (TFL)-sartorius musculocutaneous flap [7] as well as the ALT-SCIP flap [8]. These flaps, variously termed as ‘siamese’ or sequential flaps, however, are still relatively limited by their length. Sequential combinations of double flap transfers with double source vessels and distal vascular supercharge are known to provide sufficient nourishment for large skin territories. Transposing the perforator flap concept onto this allows for a more refined approach. In 2001, the authors summarized and classified the concept of combining tissue transfers [9,10]. Since that time, additional permutations and combinations of microsurgical options have been proposed. In particular, the perforator flap technology provides a less invasive procedure with reduced morbidity due to muscle preservation. It is also aesthetically superior to its predecessors, e.g., the LDMC-groin flap. This was the premise for the development of the triple TAP-ICAP-SCIP mega-flap for coverage of longer and wider defects in the extremities.
Hallock first reported the original chimera combined flap without an additional free-flap [11], while the authors expanded on this in their classification of chimeric flaps using a single source vessel [9,10,12-14] (Figure 3). The one-stage chimeric combination of an anterolateral thigh flap with a vascularized iliac bone or fibula [12-14], reported originally by us, is an example. However, the underlying premise in all of these flap constructs is that they are essentially ‘flow-through’ flaps (vascularly in-series). These combinations have been noted to have an increased risk of distal flap necrosis [15] as well as prolong the operating time necessary to harvest flaps from different donor sites in cases with very long and narrow defects. In this regard, the multi-stage combined flaps are ideal, as stratifying the overall procedure into modular components allows for shorter procedures at each stage.
The name ‘Orochi’ is derived from a mythological Japanese eight-headed snake, akin to a ‘hydra’. The term alludes to the creation of parallel vascular systems (flaps), but are connected to a single or several source vessels (Figure 3). This preserves the source vessel particularly important, e.g., in the case of the femoral vessels to the lower limb, while also minimising the number of critical microvascular anastomoses. This improves distal flap perfusion, obviates the need for the distal free-flap to be dependent on a flow-through flap, and is more physiologically in line with tissue vasculature (vascularly in parallel).
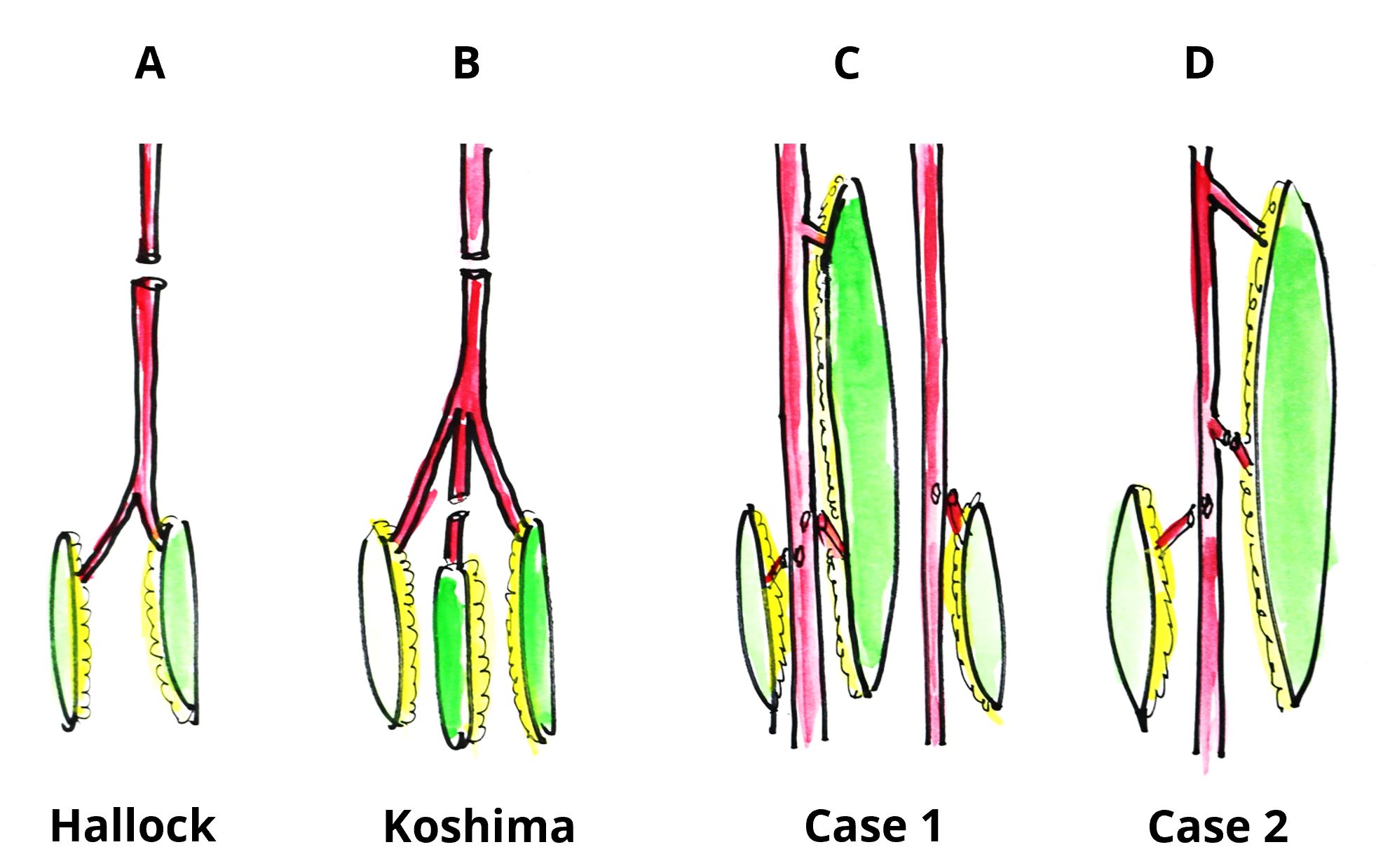
Figure 3. Classification of free combined tissue transfer. (A) Classical chimera flap (Hallock). (B) Single stage chimera flap with additional free-flap based on a single recipient vessel (Koshima). (C) Triple stage ‘Orochi’ flap. Triple mega-flap with double flaps based on double source vessels as seen in Case 1. (D) Two-stage ‘Orochi’ flap. Triple mega-flap with a flap with a single source vessel as seen in Case 2.
This is demonstrated here by the two or three-staged procedure using a free TAP flap followed by a sequential triple TAP-ICAP-SCIP mega-flap (Case 2, additionally supercharged) and free SCIP flap (Case 1), both of which are in parallel to the main source vessel and independent of one another. The additional advantages are (1) shorter procedures, (2) the ability to modularly reconstruct defects up to 50 cm without constant intra-op positional changes, and (3) minimal donor site morbidity, i.e., a secondary defect that is closed directly without having to sacrifice functional muscles. Nevertheless, the ‘Orochi’ flap system requires complicated flap elevation, microvascular expertise, and coaptation of the transected lateral femoral cutaneous nerve in certain instances [2]. It should hence be performed with much pre-op planning and meticulous dissection. In addition, this triple mega-flap is not indicated for patients who received previous axillary or groin dissection.
Regarding our multistage procedure for combined tissue transfer, it still exists that the basic advantage of the combined flap reconstruction is to cover large defects in a single-stage procedure. When we proposed the one-stage combined tissue transfer until 2000, we thought one-stage was the best. However, in the last 10 years, my initial opinion has changed with long periods of experience of lots of one-stage combined chimera flap transfers, because the one-stage combined flap transfer could not exceed beyond the standard outcome. This paper introduces the preliminary my own new concept of new style combined tissue transfers to aim aesthetic reconstruction for massive complex defects. Now, I am continuously writing series of new style multi-stage combined tissue transfers for future advancement for aesthetic microsurgery.
Regarding Case 2, there was a criticism that the aesthetic outcome in terms of contouring on the leg could be more consistent with a single-stage free-flap procedure using supercharging DIEP flap, avoiding a patchwork flap look. This method is more common and we also have many experiences in breast reconstruction. The actual defect in Case 2 was 80 cm in length. Double DIEP flaps longer than 50 cm seem to be difficult in oriental patients. Direct closure of donor site is impossible in 80 cm length. Bilateral SCIP may have the potential to extend the 80 cm length. Also, our triple mega-flap is new and has more potential to extend the territory in future. This may be a goal of the next step flap advancement.
The creation of parallel flap systems along an extremity defect, for example, allows its use for extra long (up to 50 cm) and wide defects, especially useful in young females and children. As any subsequent flap reconstruction uses the source vessel as a hub rather sacrifices its primary function, the ‘Orochi’ flap concept allows any number of flaps to be added onto the vascular axis without compromising distal vascularity of both the sequential flap construct or the extremity/organ.
Regarding another ‘Orochi’ flap, combined sequential (ALT-SCIP, DIEP-SCIP, DIEP-DIEP, SCIP-SCIP, TAP-DIEP flaps etc.) and additional free-flapsare proposed. The indications of these flaps are defects with difficult repair with one-stage chimera transfer with respect to fewer techniques, manpower, and/or surgical time; multistage tissue transfer for extensive 3-dimensional complex defects including longer and wider defects, not only in the limbs, but the whole body.
We believe that the concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel) exemplified here would open a new vista in reconstructive microsurgery.
Received date: October 08, 2018
Accepted date: December 24, 2018
Published date: March 20, 2019
The authors wish to thank Professor Rryukiti Kawamura, Chief of the Department of Dermatology of Yamanashi University in Japan for his support in this study.
The work was presented in part at the Japanese Society of Reconstructive Microsurgery in Niigata, Japan on November 11, 2011 as well as the Maliniac Lecture in the American Society of Plastic Surgery in Chicago, USA on October 13, 2014.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2019 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The senior author (Dr. Isao Koshima) designed a tibial osseo-periosteal (TOP) flap. TOP flap has a favorable anatomical position with a thin skin around it, hence it is a good option for an island flap. TOP flap can be used for various mild to moderately sized osteo-cutaneous defects with low morbidity. In this article, the authors describe their experience of the first reported cohort of TOP flaps in clinical practice.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
Koshima I, Imai H, Yoshida S, Harima T, Mizuta H, Yamashita S, Nagamatsu S, Yokota K, Kannan R. The ‘Orochi’ flap concept: Multi-stage combined flap using sequential flaps. Int Microsurg J 2019;3(1):4. https://doi.org/10.24983/scitemed.imj.2019.00108