With recent advances in understanding molecular pathogenic cytokine network of psoriasis, several innovative biologic drugs have been successfully delivered to the market for treatment of various forms of psoriasis. Indeed, it becomes a hot spot in developing biologics that targets proinflammatory cytokines for autoimmune diseases. However, during long period of time evolution, our body establishes a sophisticated feedback mechanism to ensure equilibrium of our genetical system. This author concerns that long-term application of biologics will destroy the natural balance, and argues to rethink about small molecules that can restore immune homeostasis, thereby naturally correcting overproduced proinflammatory cytokines, as a strategy of new drug development against psoriasis and other autoimmune disorders.
Psoriasis is a chronic inflammatory disease affecting up to 3% or 7.5 million Americans [1,2] and 125 million people worldwide [3], of which more than 1.5 million Americans are considered to have a moderate-to-severe form of the disease. Up to 30% of the psoriatic patients may also suffer from seronegative spondyloarthritis [1]. Moreover, inflammatory mediators associated with psoriasis may increase the risk of obesity, diabetes, thrombosis, and atherosclerosis [4].
Conventionally, psoriasis can be treated with compounds that topically or systemically inhibit inflammation, cell proliferation, or cell differentiation. Various topical and systemic therapies include anti-inflammatory agents (e.g., glucocorticoids), analgesics, chemically synthesized disease-modifying antirheumatic drugs (DMARDs; e.g., methotrexate and ciclosporin), and antiproliferative agents (e.g., retinoids and vitamin D analogs). However, efficacy of these therapies is generally not satisfactory.
Psoriatic disease is generally accepted as an autoimmune disease. Accumulating evidence suggests that autoimmune diseases/inflammations are the result of an imbalance of immune system. Naïve T cells, when stimulated by their cognate antigens in a suitable cytokine environment, can differentiate into at least four major helper T cell lineages (Th) that play a central role in orchestrating the immune system response. The four major lineages are Th1, Th2, Th17, and Treg cells (CD4+/CD25+/forkhead box P3 [Foxp3+]). These subsets are defined by the signature cytokines they produce and the functions to which they give rise.
The presence of these subsets must be continually fine-tuned to maintain equilibrium of the immune system (homeostasis) and avoid inflammatory diseases. Critically, a balance between pro-inflammatory (Th17 and Th1) and anti-inflammatory T cells (Treg and Th2) is required, which are homeostatically controlled with positive and negative feedbacks.
Like many other autoimmune diseases, psoriatic lesions likely evolve interplay between cells and mediators of the immune system. Initial triggers by multiple environmental factors such as physical trauma, UV or bacterial products, start a cascade of events, including the production of TNF-α, interferon-α, interferon-γ, IL-1β, and IL-6 by innate immune cells, which leads to the activation of myeloid dendretic cells (DCs). These activated DCs migrate into draining lymph nodes, present antigens to T cells, and secrete mediators, for example IL-12 and IL-23, causing the differentiation of naïve CD4+ lymphocytes into effector T cells, such as Th17 and Th1. In turn, these effector cells recirculate and slowdown in skin capillaries in the presence of selectin-guided and integrin-guided receptor-ligand interactions [5]. Subsequently, effector T cells secrete proinflammatory mediators such as IL-17A, IL-17F, interferon-γ, and TNF-α that activate keratinocytes, leading to the production of antimicrobial peptides, inflammatory cytokines, chemokines, and S100 proteins, which feed back into the disease cycle and shape the proinflammatory infiltrate [5]. Recent research indicates that both IL-23 and IL-17 play important roles in the development of psoriatic plaques [6-9]. IL-17 produced by Th17 cells and others, plays an important role in the regulation of the adaptive and innate immune systems as well as the pathogenesis of psoriasis. IL-17 has been associated with neutrophil recruitment, induction of a Th2 response, stimulation of macrophages to produce IL-1β and TNF-α, and induction of inflammatory mediators such as matrix metalloproteinases (MMPs) [10]. On the other hand, IL-23, with the help of IL-6 and transforming growth factor (TGF)-β, stimulates the differentiation of T cells to Th17 cells and plays an integral role in the survival of these cells, which consequently leads to an increase in the release of IL-17 [7].
Based on these critical pathological findings on psoriasis, there are at least two strategies in developing effective drugs against psoriasis: (1) blocking inflammatory mediators using antibodies, such as IL-17, IL-23, or TNF-α or other inflammatory cytokines; (2) restoring normal skin homeostasis using small molecules to naturally correct the overproduced inflammatory mediators.
Indeed, several innovative drugs become available on the market for treatment of psoriasis. They are all biologics as antibodies either against IL-17 antibody, such as ixekizumab from Eli Lilly or against IL-23, such as guselkumab from Johnson & Johnson, or other proinflammatory cytokines. It appears these new drugs are effective and tolerable in short run [11,12]; long term pharmacovigilance, however, is warranted.
Besides expensive and inconvenient to use the biologics, this author concerns that long-term application of biologics will destroy the natural balance of the immune system, and argues to rethink about small molecules that can restore immune homeostasis, thereby naturally correcting overproduced proinflammatory cytokines, as a strategy of new drug development against psoriasis.
Biologic Drugs Worsen Imbalanced Physiological System
Mechanically, all biologic drugs to treat psoriasis work through neutralizing/blocking a protein, such as IL-17 or IL-23. They are indeed so powerful that they can completely block activities of IL-17 or IL-23 functions, including their normal physiological functions. Since psoriasis is caused by imbalanced immune system, while these biologic drugs work well to relieve disease symptoms, they, however, make imbalanced immune system worse. Consequently, these treatments suffer from long-term disadvantages including severe side effects (induction of cancer and infections because of completely blocking physiological functions of IL-17 or IL-23), and critically when the treatments stop, all disease symptoms will not only immediately rebound back, but the disease conditions will often get much worse as balance of your immune system becomes deleterious, it do occur clinically (personal communications). The observed development of ulcerative colitis and Crohn during ixekizumabe trial [11,12] is another good example of deleterious immune system by the biologic.
During long period of time evolution, our body establishes a sophisticated genetical system, which is fine tuned and highly regulated. Feedback mechanism is a general mechanism in managing gene expression. To be short, it says when a gene is activated to transcript to mRNA, and the mRNA then is translated into corresponding protein, the protein however will send a negative signal to its gene to terminate the gene expression when it reaches necessary amount. In contrast, when the level of corresponding protein is too low (neutralized in case of using antibody), it will send a positive signal to activate/enhance gene transcription. The feedback mechanism plays a crucial role in dynamic gene expression in nature [13].
Stapleton et al. [13] provided an example to show how feedback mechanism works. Authors used an RNA/protein interaction-based synthetic translational switch to create a feedback system that tightly controlled the expression of proteins of interest in mammalian cells. In this case, feedback is mediated by modified ribosomal L7Ae proteins, which bind a set of RNA motifs with a range of affinities (Figure 1).
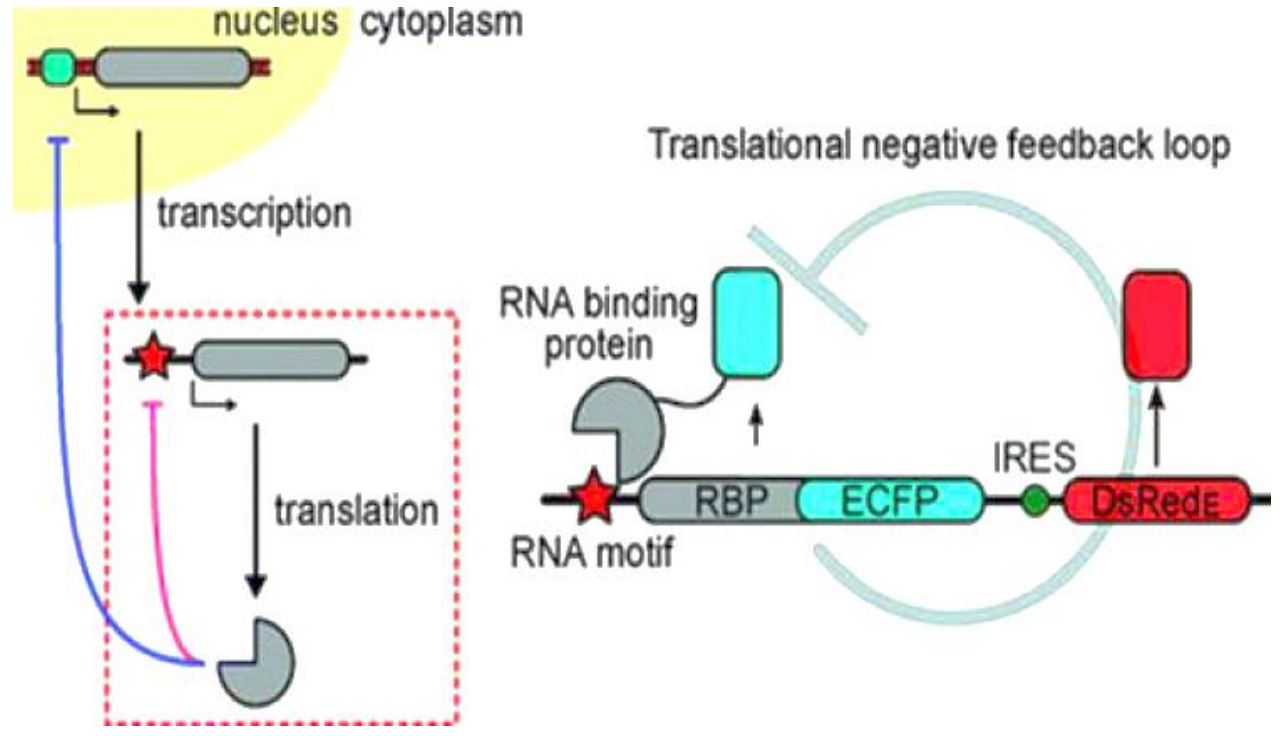
Figure 1. Example of feedback mechanism in L7Ae gene transcription and translation. Feedback is mediated by modified ribosomal L7Ae proteins, which bind a set of RNA motifs with a range of affinities. Newly translated L7Ae binds its own mRNA, inhibiting further translation. This inhibition tightly feedback-regulates the concentration of L7Ae and any fusion partner of interest. The L7Ae protein can simultaneously and tunably regulate the expression of multiple proteins of interest by binding RNA control motifs built into each mRNA, allowing control over the coordinated expression of protein networks [13].
Newly translated L7Ae binds its own mRNA, inhibiting further translation. This inhibition tightly feedback-regulates the concentration of L7Ae and any fusion partner of interest. A mathematical model predicts system behavior as a function of RNA/protein affinity. Authors further demonstrated that the L7Ae protein could simultaneously and tunably regulate the expression of multiple proteins of interest by binding RNA control motifs built into each mRNA, allowing control over the coordinated expression of protein networks [13].
The same principle applies to gene expression of IL-17 or IL-23 or any other gene. In case of IL-17, as shown in Figure 2, when the gene is activated, it will be transcribed into mRNA, and then translated into IL-17 protein. The expression process will be terminated when appropriate amount of IL-17 protein is made because of negative feedback regulation. However, this equilibrium is destroyed when the gene product, i.e. IL-17 protein is constantly and completely neutralized by its antibody (the drug treatment). The depletion of IL-17 protein will send a strong positive signal to the IL-17 gene (positive feedback), which will be then overwhelmingly transcribed into mRNA. Therefore, treatment of anti-IL-17 or IL-23 will make the level of corresponding mRNA skyrocket high, thereby worsening the imbalanced immune system. That is why the disease symptoms immediately rebound back and often get worse when the treatment of these biologic drugs is terminated.
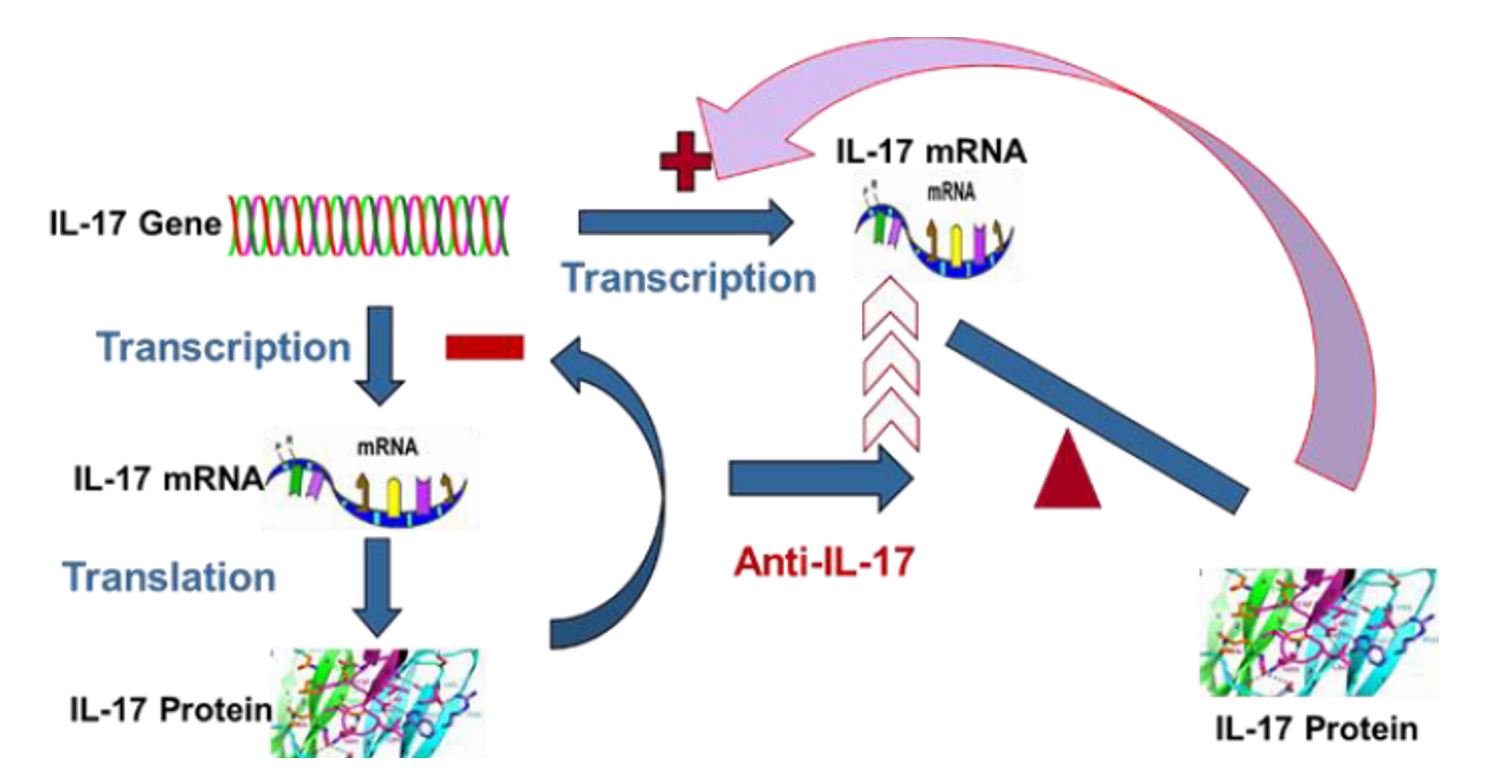
Figure 2. Scheme of feedback control of IL-17 Expression: Under normal physiological condition, IL-17 gene will be transcribed into its mRNA, and then the mRNA is translated into the IL-17 protein. The gene expression will be slowdown or stopped when the product IL-17 protein reaches necessary amount through negative feedback mechanism. However, the natural equilibrium of the gene expression will be destroyed when anti-IL-17 is used, which strongly or completely neutralized IL-17. The complete depletion of IL-17 protein will send a strong signal (positive feedback) to make the gene overwhelmingly produce mRNA. Consequently, the mRNA level of IL-17 will be constantly at very high level, which will be translated into huge amount of IL-17 protein leading to disease symptoms immediately rebounded back or even getting worse when the treatment is terminated.
In addition, as Th17 cell is also a key player in protection against microbe invaders, infection is unavoidable event in using blockers of IL-17 to treat psoriasis. Indeed, various infections have been observed clinically, which include, but are not limited to, infections of the upper respiratory tract and TB [11,12]. It has been also reported that Ixekizumab induces nonmelanoma skin cancers (NMSC), and inflammatory bowel diseases (both ulcerative colitis and Crohn’s disease) – another good example of imbalanced immune system worsening. Evaluation of long term therapeutic benefits and risk (pharmacovigilance) of biologic drugs is warranted [11,12].
Small Molecule Restoring Homeostasis of Immune System Thereby Correcting Disease Conditions
Recent studies demonstrate that AhR signaling play a critical role in maintaining immune homeostasis [14-17]. Since the skin is a highly sophisticated sensory organ covering the surface of the body, AhR mediated maintenance of skin homeostasis in response to external physical and chemical stimuli plays key roles in self-dense [15].
It is now clear that aryl hydrocarbon receptor (AhR) signaling plays critical role in the biological response to many environmental pollutants, which provides important example of mammalian organisms adapt to certain classes of hazardous chemicals [18]. Most importantly, in recent years, interest in AhR biology has grown beyond a toxicological scope as research has found a physiological role for this receptor in normal development. As a result, the study of AhR pharmacology has been paid much attention as investigators are able to differentiate the two different types of AhR ligands, endogenous and exogenous ligands that play different roles in the regulation of immunological response. It becomes clear that endogenous ligand-AhR signaling is in favour for Treg cell differentiation, thereby preventing reactivity to self-proteins and ensuing autoimmunity [19-21]. For example, kynurenine, an endogenous AhR ligand and the first breakdown product of tryptophan in the IDO pathway and 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), interacts with the AhR in T-cells and enhances the differentiation of Tregs that reduce an immune response when inflammation occurs [20]. When Treg cells reach necessary amount, kynurenine interacts with the AhR in DCs to enhance the generation of IDO, leading to further tryptophan break down. This response is self-limited, as once the tryptophan in the inflammatory milieu is dissipated, kynurenine is no longer generated and Treg generation is decreased [19].
In contrast to the role that the endogenous ligand-AhR plays in regulating autoimmunity, at interface organs such as the lung, gut, and skin, exogenous ligand exampled as environmental pollutants-mediated AhR activation leads to an effector response [21]. At these locations, toxins or environmental pollutants binding to the AhR in immune cells lead to Th17 differentiation, thereby producing inflammatory response.
Therefore, AhR pathway is a promising therapeutic for diseases of nervous, liver, and autoimmune diseases through AhR ligand-mediated interventions and other perturbations of AhR signaling [22].
NTI528 is a Unique Endogenous AhR Ligand and Restore Immune Homeostasis
NTI528 is a small molecule developed by Natrogen Therapeutics International, Inc. Recent studies have discovered that NTI528 is a unique endogenous AhR ligand and shares various common biological characteristics of endogenous AhR ligand activities [19-21,23,24], such as potently activates CYP1A1 expression, inhibits differentiation of Th17, induces differentiation of Treg, and IL-22. It has been also demonstrated that NTI528 inhibits the expression of IL-1β, IL-6, TNF-α and stimulates IL-10 in activated human monocytes in vitro. A small proof-of-concept clinical trial using NTI528 topical cream in treating moderate-to-severe psoriasis was conducted to evaluate its efficacy and safety.
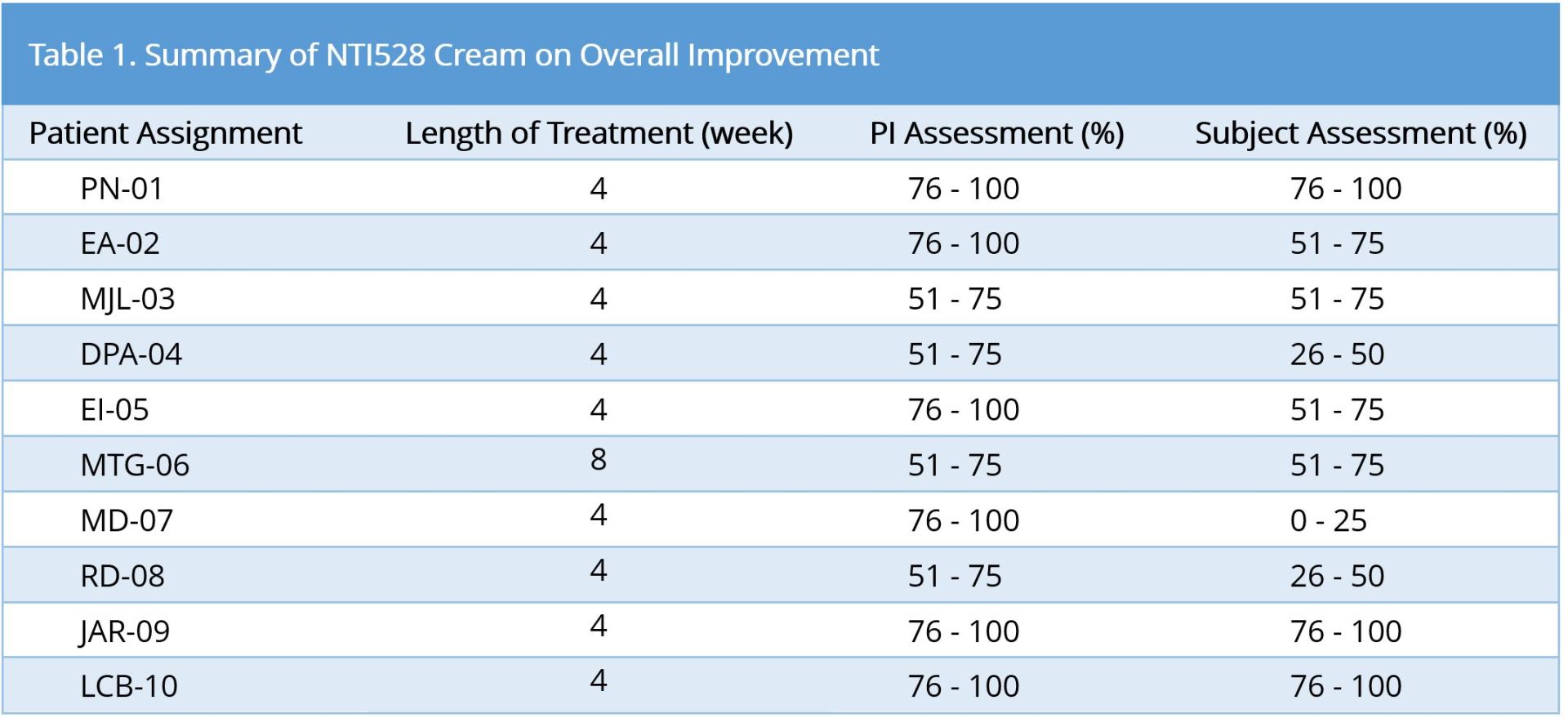
A total of 10 healthy men and women were enrolled in the study. All patients were 21 years or older and had moderate-to-severe plaque psoriasis covering at least 5cm2 of their body. After the patient had ample opportunity to review, ask questions, and sign the IRB-approved consent form, eligibility screening and a medical history were taken. The Principal Investigator (PI) rated the severity of the plaque psoriasis to ensure the patient’s eligibility for participation. Each eligible patient was photographed and given enough NTI528 topical cream to last for 28 days (+/- 3 days) with twice-a-day use. At the 28-day visit the patient was assessed for overall improvement in psoriasis. If the PI determined that the improvement was greater than 50% (51% to 100%), the patient’s enrolment in the study and all end-of-study paperwork was completed. If a patient’s response to treatments was 50% or less (0% to 50%), their enrolment would continue for an additional 4 weeks.
Efficacy was evaluated by the following criteria: quartile scale: 0-25%, 26-50%, 51-75%, 76-100%; severity scale (itching, redness, and scaling): 0 = None, 1 = some, 2 = Moderate, 3 = Severe, 4 = Maximum; body coverage score 0 = 0%; 1 = <10%; 2 = 10-29%, 3 = 30-49%, 4 = 50-69%, 5 = 70-89%, 6 = 80-100%.
The PI measured the level of improvement in psoriasis as well as improvement in erythema, scaling and pruritis. The patient also measured the level of improvement in psoriasis as well as improvement in erythema, scaling and pruritis. The patient’s log was checked for compliance and photographs of the treatment area were taken. All adverse events (AEs) were timely recorded.
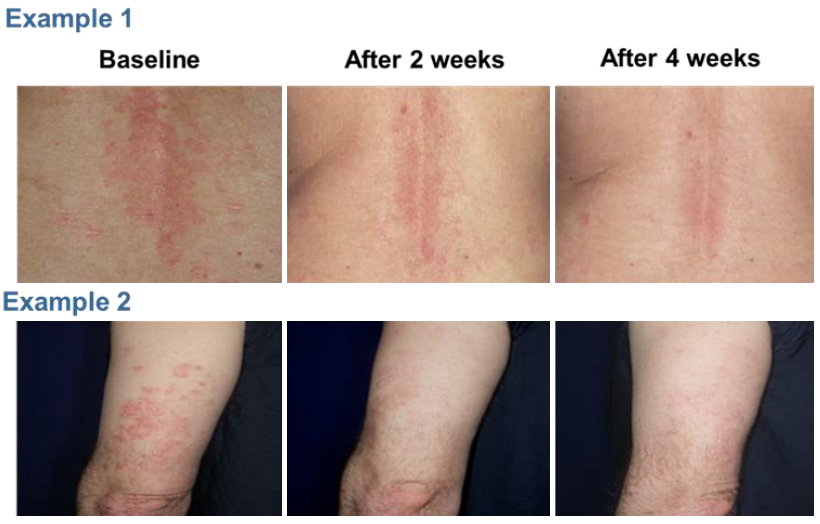
The study showed that all patients completed the study and all patients had an improvement level of at least 51%. Nine patients achieved significant and satisfactory response within 4 weeks (1 cycle) and 1 patient achieved significant satisfactory response within 8 weeks (2 cycles) (Table 1). No adverse event was reported. Figure 3 showed example photographs of patients before and after the treatment with NTI528 topical cream.
Ninety percent (9/10) of subjects finished study after 4 weeks; ten percent (1/10) of subjects finished study after 8 weeks.
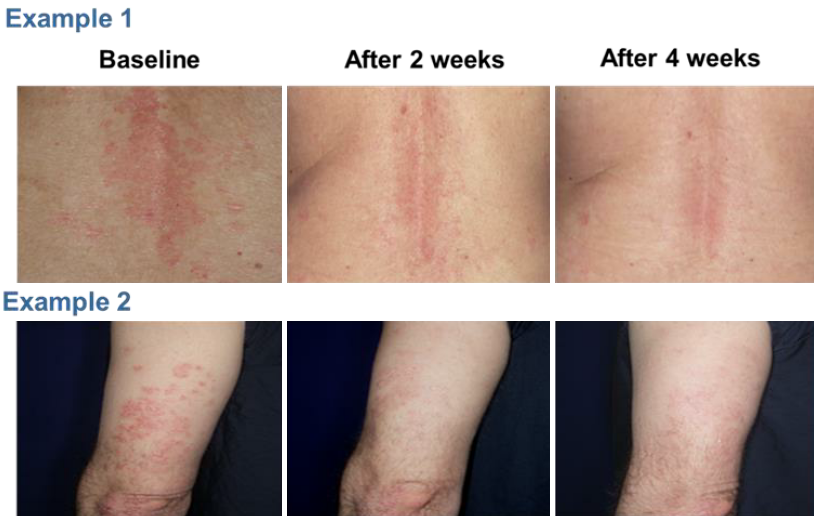
Figure 3. Example photographs of patients before and after the treatment with NTI528 topical cream.
Although scale-up clinical trials are needed to confirm its anti-psoriasis activity and safety, it does provide a valuable and promising example that developing small molecule that restores immune homeostasis, thereby highly effective, less or no safety issues, and less expensive, is a plausible strategy in treating psoriasis as well as other autoimmune disorders. Further studies are needed to confirm the inference.
Received date: August 25, 2017
Accepted date: October 16, 2017
Published date: November 14, 2017
© 2017 The Author. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
This case report shows the effectiveness of Apremilast in refractory ophiasis AA with a rapid and highly scalp-hair regrowth with a cosmetically important response.
Thanks for the interesting article. AHR and its ligands may serve as tools for modulating immune response, but I have few concerns:
Accept for publication.
This article is accepted for publication.
Wang LG. Rethinking of small molecules in treating psoriasis. Arch Clin Dermatol 2017;1(1):3. https://doi.org/10.24983/scitemed.acd.2017.00042