Objective: An increasing burden is loaded on clinical laboratories for daily testing of body fluids, including pleural fluid, peritoneal fluid (ascites), pericardial fluid, and cerebrospinal fluid. The request for detection of cellular components in the body fluid samples is usually delivered simultaneously to the hospital’s hematology laboratory and cytology laboratory. The medical technologists of hematology laboratory provide the screening result for suspicious malignant cells; meanwhile, the pathologists of cytology laboratory provide the presence of cancer cells at a diagnostic level. However, unfortunately, neither of these results reaches 100% sensitivity or specificity. To assess the clinical practice values, the present study performed a comparison of the hematology and cytology laboratories on the detection of malignancy in body fluids.
Methods: The body fluid specimens were collected either in the absence or in the presence of an anticoagulant agent, such as heparin. The finding of body fluid malignancy was remarked along with the differential count of blood cells in the report. Cytology laboratory, the body fluid specimens were subjected to cytospin centrifugation, followed by Papanicolaou stain, and then the morphological results were graded as negative, atypical, suspicious or positive for malignancy. The agreement of body fluid malignancy between the hematology laboratory and cytology laboratory was assessed by Kappa statistics and the differences between the groups were evaluated by chi-square test.
Results: A total of 248 body fluid samples, including 191 (77.0%) pleural fluids, 50 (20.1%) ascites, 4 (1.6%) pericardial fluids, and 3 (1.2%) cerebrospinal fluids were retrospectively surveyed in the National Cheng Kung University Hospital. The comparisons resulted in 105 (42.3%) positive and 106 (42.7%) negative reports of malignancy matched between hematology and cytology laboratories (Kappa agreement = 0.70). At a follow-up for 6 months, 141 fluid samples were confirmed with malignancy and 107 were without malignancy. It comprised 19 cancer types and the yielding of sensitivity, specificity, positive prediction value, and negative prediction value were 89.4%, 100.0%, 100.0% and 87.7% respectively for the hematology laboratory, and 85.8%, 99.1%, 99.2% and 84.1% respectively for the cytology laboratory. Furthermore, the hematology laboratory reported 15 false negative cases but no false positive, while the cytology laboratory reported 20 false negative cases and 1 false positive.
Conclusion: The hematology laboratory equipped with well-trained laboratory technical staff could provide early reporting service on body fluid malignancy, which might hold a potential reaching comparable competency similar to the cytology laboratory.
Body fluids, including pleural, peritoneal (ascites), pericardial, and cerebrospinal effusions, consist of testing parameters which provide diagnostic values by examining the biochemical analytes and cellular components, such as infiltrating benign and malignancy cells [1,2]. The evaluation of the cellular compositions in these specimens is performed in either the hematology laboratory or the cytology laboratory or both, on the samples which were concomitantly collected but independently processed. The hematology expertise of medical technologists provides primarily reports based on absolute and differential hematological cell counts in the paired specimen of body fluid and peripheral blood, and screens for any suspicious malignant cells of other tumor types along with hematological lineage of differential precursors. Meanwhile, the cytology expertise of pathologists centrally checks for the presence of malignancy at a diagnostic-level rather than at a screening-level, yielding various sensitivities as reported; for example, an average of around 70% in detecting malignant cells in pleural fluid [3] and 40-65% in peritoneal fluid [4]. The diagnostic yields for malignancy by cytology might vary due to personnel skills and likewise depend on tumor and effusion types [5,6]; for example, a high sensitivity of > 95% in carcinoma in peritoneal fluids [4], but low sensitivities of < 50% in lymphomas, and < 25% in sarcomas and mesotheliomas in pleural fluids [3].
Closely distinct sample processes of body fluid in the hematology and cytology laboratories are applied with sodium citrate or heparin serving as anticoagulants and the cytospin methodology to concentrate scant cells. The medical technologists in the hematology laboratory examine the cells which are widely stained with Romanowsky dyes (Wright, Giemsa and Liu’s stain) [7,8], whereas the pathologists in the cytology laboratory perform morphological observation upon the cells with Papanicolaou stain [9]. Furthermore, the hematology laboratory runs for 24 hours per day and usually presents the screening reports earlier than the diagnostic reports from the cytology laboratory on the sample duplicates. Since the body fluid malignancy has not yet been fully detectable merely by cytology laboratory service, it is worthy to investigate the possibility of compensation with the results from the hematology laboratory. In this study within a hospital, the performance for finding positive body fluid malignancy in the hematology laboratory was evaluated and compared with that of the cytology laboratory.
Study Design
This study was designed retrospectively to assess and compare the performance of detecting body fluid malignancy by the hematology laboratory and the cytology laboratory of National Cheng Kung University Hospital (NCKUH). The cases were recruited from the consecutive body fluid specimen duplicates submitted for testing in the NCKUH hematology and cytology laboratories during 2015 to 2016. The exclusion criteria were the specimens that were inadequate for both examinations, and the cases that were incomplete for follow-up. The types of body fluid and reports of body fluid malignancy were recorded independently. The final diagnosis on the patient’s malignancy was then combined with the reports of further diagnostic services with positron emission tomography, computed tomography, histological examinations of biopsy, and bone marrow aspiration, as requested thereafter within 6 months. The study was approved by the Institutional Review Board of National Cheng Kung University Hospital vide no. B-ER-107-06 for data collection and analysis under waived informed consent from individual patients.
Morphological Examination and Reporting
The body fluid specimens were collected either in the absence or in the presence of an anticoagulant agent, such as heparin. In NCKUH hematology laboratory, the samples were analyzed with automated complete cell counter (LH750, Beckman Coulter, Brea, CA) to determine the absolute and differential blood cell numbers; meanwhile, also mounted on a slide by cytospin centrifugation (Shandon cytospin 3, Thermo Scientific, Waltham, MA) and then visualized under microscopy with Liu’s stain to determine the hematological lineage of differential stages of blood cells and concomitantly with other solid-cancer originated malignant cells. The finding of body fluid malignancy was remarked along with the differential count of blood cells in the report. In NCKUH cytology laboratory, the body fluid specimens were subjected to cytospin centrifugation, followed by Papanicolaou stain, and then the morphological results were graded as negative, atypical, suspicious or positive for malignancy.
Statistical Analysis
The agreement of body fluid malignancy between the NCKUH hematology laboratory and cytology laboratory was assessed by Kappa statistics and the differences between the groups were evaluated by chi-square test. The statistical analysis was conducted using SPSS software (V.19.0; SPSS, Chicago, IL).
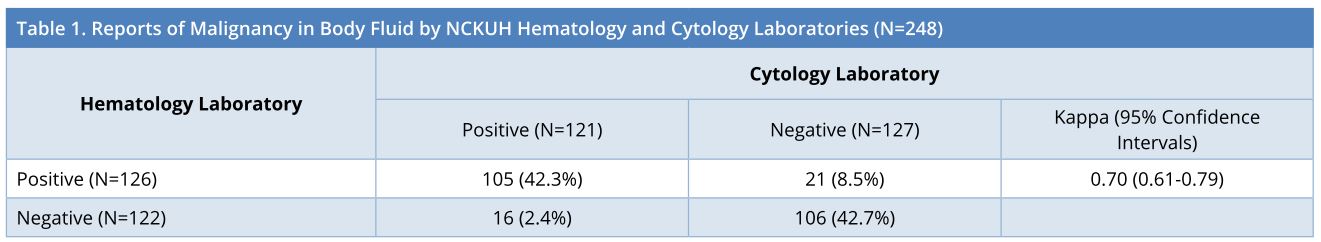
In a total of 248 body fluid samples, the matched reports of malignancy were 105 (42.3%) positive and 106 (42.7%) negative in both hematology and cytology laboratories. Discrepancy was shown in 21 (8.5%) samples which were hematology-positive/cytology-negative and in 16 (2.4%) samples which were hematology-negative/cytology-positive. The results shown in Table 1 suggested that the reports of malignancy from NCKUH hematology laboratory and cytology laboratory might agree substantially (Kappa = 0.70, 95% confidence intervals = 0.61-0.79).
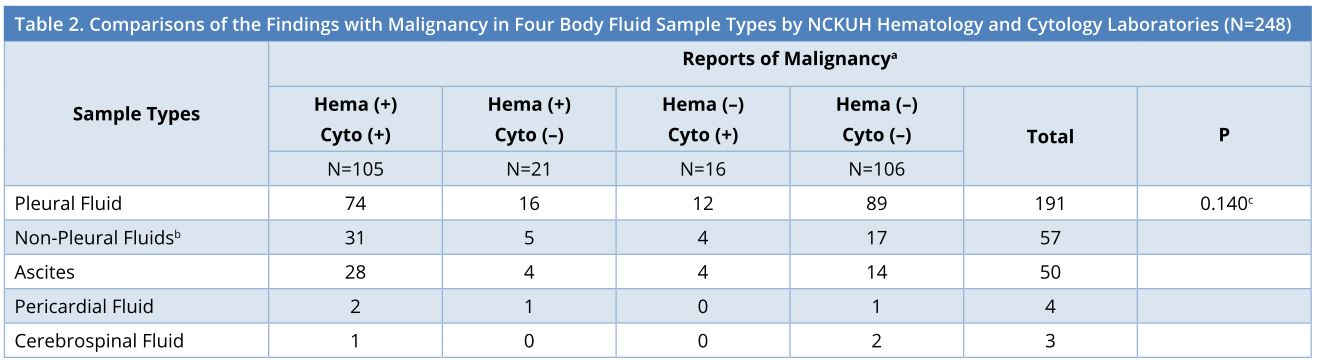
As stratified by sample types, the 248 effusions consisted of 191 (77.0%) pleural fluids, 50 (20.1%) ascites, 4 (1.6%) pericardial fluids, and 3 (1.2%) cerebrospinal fluids. The breakdown findings with malignancy in the 4 sample types by NCKUH hematology laboratory and cytology laboratory were displayed in Table 2, showing no significant differences between the groups of pleural fluid and non-pleural fluids (P = 0.140).
The patients were then checked within 6 months by the other diagnostic services of positron emission tomography, computed tomography, histological examinations of biopsy, and bone marrow aspiration to confirm malignancy. Among the total of 248 patients, malignancy was confirmed in 141 and 107 were without malignancy. The yielding of sensitivity, specificity, positive prediction value and negative prediction value were 89.4%, 100.0%, 100.0% and 87.7% respectively for the hematology laboratory, and 85.8%, 99.1%, 99.2% and 84.1% respectively for the cytology laboratory, as shown in Table 3. The results (Table 3) displayed that the NCKUH hematology laboratory reported 15 false negative cases but no false positive, while the cytology laboratory reported 20 false negative cases and 1 false positive. Taken together, the survey in this study indicated that the NCKUH hematology laboratory could reach a comparable diagnostic competency similar to that of the cytology laboratory.
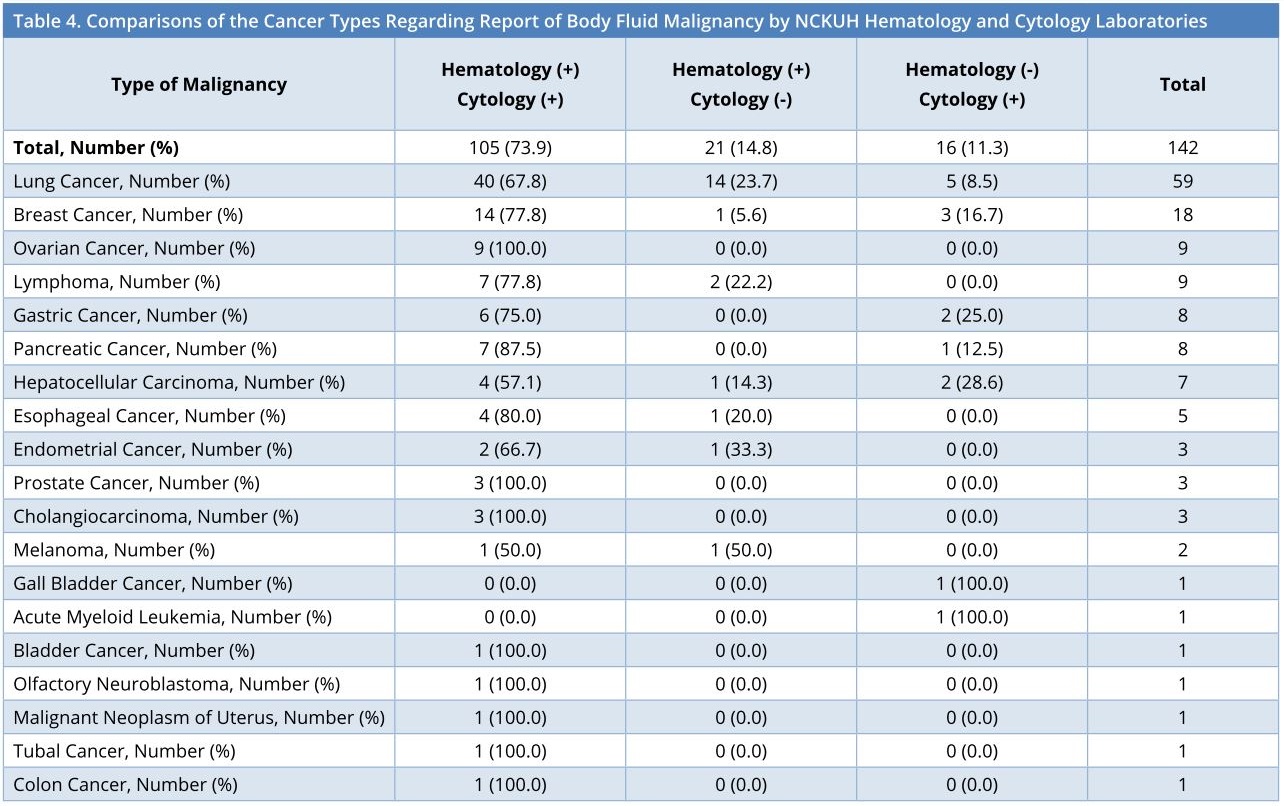
This study comprised 19 types of malignancy, showing the top two cancers originating from lung and breast. As displayed in Table 4, no significant differences existed between the cancer types among the reports of body fluid malignancy by the NCKUH hematology laboratory and cytology laboratory. Additionally, pleural effusions were dominant in the diagnosis in the order of lung cancer, breast cancer, lymphoma, pancreatic cancer, and esophageal cancer, whereas ascites were dominant in the order of ovarian cancer, gastric cancer, hepatocellular carcinoma and esophageal cancer, as shown in Table 5.
NCKUH, National Cheng Kung University Hospital.
NCKUH, National Cheng Kung University Hospital.
a Hema, from hematology laboratory; cyto, from cytology laboratory.
b Non-pleural fluids consist of ascites, pericardial fluid and cerebrospinal fluid.
c Comparison between the groups of pleural fluid versus non-pleural fluids.
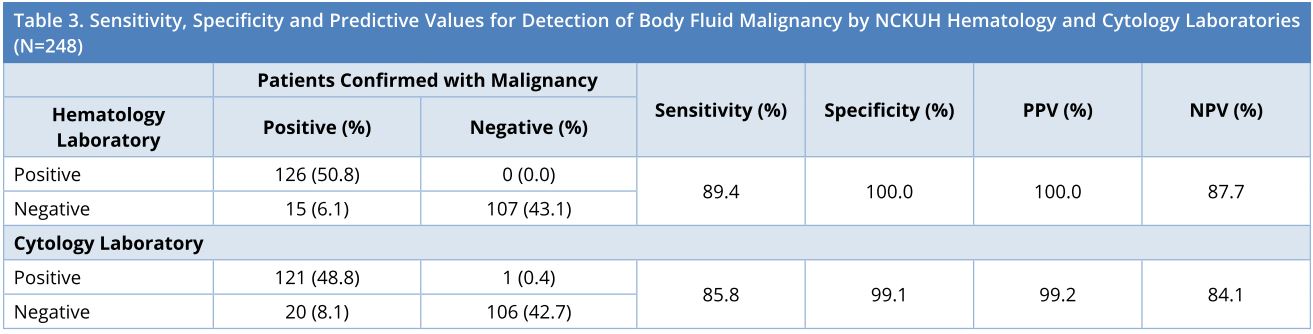
NCKUH, National Cheng Kung University Hospital; NPV, Negative Predictive Value; PPV, Positive Predictive Value.
NCKUH, National Cheng Kung University Hospital.
Increasing clinical testing requests on body fluids represent diagnostic values, which reflect the differential bio-tracer of the local sampling site. However, the analysis features with a stressful burden because the body fluid compositions and the processing methods largely vary between fluid types [1]. Besides, even the regulatory compliance for complete blood count automation in the hematology laboratory is frequently not optimized for body fluid samples. The microscopic morphological observation of body fluid malignancy by manual methods, therefore, depends on the expertise of well-trained and experienced staffs. A substantial number of body fluid duplicate samples are simultaneously delivered to the hematology and cytology laboratories. The examinations on body fluids in the hematology laboratory include physical parameters, chemical analytes, blood cell count, and lineage differentiation, mainly with a purpose to differentiate transudates versus exudates [10], and concomitantly to screen for morphologically aberrant cells. Meanwhile, the cytology laboratory aims to examine the presence of malignancy at a diagnostic level. The results of hematology body fluid routine are requested in less than 12 hours, but it takes 3 days for cytology laboratory reports, which likely represent the “gold standard”. In this study, the first-line screening of body fluid was re-visited in the hematology laboratory. Nevertheless, the hematology laboratory currently reached comparable sensitivity and specificity as achieved by the cytology laboratory for the detection of body fluid malignancy. The results, therefore, suggested that the hematology laboratory might provide reports which are as valuable as those from the cytology laboratory, not only for screening but also for diagnostic orientation. Since the hematology laboratory is quicker in reporting positive results, it is promising to establish a real-time alarming rule with the cytology laboratory within a hospital.
The diagnostic ability of hematology laboratory in detecting body fluid malignancy has been compared with that of cytology laboratory, yielding less satisfactory sensitivities ranging from 23% to 64.9% in the previous studies [6,11,12], whereas an elevated sensitivity of 89.4% has been achieved in our current evaluation. Sample and cancer types, stain of specimen, and personnel education might contribute to the outcomes of detecting body fluid malignancy. In our study population, the pleural fluid samples outnumbered ascites, but it was vice versa in the other studies [6]. The Romanowsky Liu’s stain was used routinely to visualize hematological cell morphology in the NCKUH hematology laboratory while Wright stain [13] in the other. Nevertheless, the carcinoma types in Taiwan local area might account for the present diagnostic yields, as suggested previously [5,6]. Besides, the highly qualified personnel techniques in the NCKUH hematology laboratory might also be one determinant factor that confers diagnostic competence of body fluid malignancy. In Taiwan local area, the medical technologists receive a 4-year full-term education comprising academic-based and laboratory professional courses and undergo clinical hospital practice at the university level before obtaining the national license for an entry into the real-world services. Furthermore, regular continued education and skilled training resumption in morphological identification are obligated on a per year basis. Concomitantly, the previous results demonstrated that the sensitivity of detecting body fluid malignancy could be elevated from 23.4% to 60% after an implant of the quality assurance plan with educational components [6]. Taken together, the diagnostic values in body fluid malignancy can be increased by improving the abilities of hematology laboratory’s technologists in identifying cell morphology.
Currently, the increasing aged population might give rise to cancer incidence [14]. Colon cancer, hepatocellular carcinoma, and lung cancer are the top leading malignancy for males, whereas breast cancer, colon cancer, and lung cancer for females in Taiwan. The four common types of cancer together account for more than 50% of the overall cancer illness [11]. In this study, most of the body fluid malignancy came from lung cancer and breast cancer cells, which primarily exfoliated in pleural effusions and secondarily in ascites. It was shown that the body fluid analysis could establish the diagnosis of metastatic adenocarcinoma in > 70% of cases, which was much higher than the reported sensitivities for mesothelioma (10%), squamous-cell carcinoma (20%), sarcoma (25%), and lymphoma (25-50%) [4]. Therefore, the predominant malignant types of lung and breast cancers involving the pleural fluid might be attributed to the high sensitivity and comparable detection in the NCKUH hematology and cytology laboratories.
The limitations of the study included that it was conducted with cases with query for testing in both the NCKUH hematology and cytology laboratories; and the numbers of several cancer types were small in the context of the majority pleural fluid specimens.
The hematology laboratory when providing well-trained competences might potentially be comparable to the cytology laboratory for a diagnosis of body fluid malignancy.
Received date: April 24, 2018
Accepted date: June 20, 2018
Published date: August 18, 2018
None
None
The manuscript was presented at a meeting: 9th Asia-Pacific Forum of Medical Laboratory Sciences 2017, Taiwan Association of Medical Technologists (TAMT), Taoyuan, Taiwan, held on April 15-16, 2017.
The authors would like to thank Ting-Yu Hou for her assistance in the manuscript preparation.
© 2018 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The author presents a case of BCC developed on an active lesion of mucocutaneous leishmaniasis as one of many cases of skin cancer frequently seen at the Regional Leishmaniasis Control Center (RLCC) clinics in Yemen.
Patients with gynecological abdominal wall malignancies can benefit significantly from radical resection and autologous reconstruction. The pedicled anterolateral thigh flap is the preferred donor site, offering a reliable solution to abdominal wall reconstruction in this setting. The satisfactory results should prompt a more aggressive surgical approach for these patients. This article describes the authors' experiences with the abdominal reconstruction following surgical resection of gynecological abdominal wall malignancy using pedicled anterolateral thigh flap.
|
Kappa |
Agreement |
|
0.01–0.20 |
Slight Agreement |
|
0.21– 0.40 |
Fair Agreement |
|
0.41–0.60 |
Moderate Agreement |
|
0.61–0.80 |
Substantial Agreement |
|
0.81–0.99 |
Almost Perfect Agreement |
After reviewing the revised manuscript, I am satisfied with the authors' responses and the corrections in the manuscript.
I accept the revised manuscript for publication.
Lin SI, Tseng JY, Chang KC, Young KC. Detection of malignancy in body fluids: A comparison of the hematology and cytology laboratories. J Appl Clin Pathol 2018;1(1):5. https://doi.org/10.24983/scitemed.jacp.2018.00077