Objectives: Gynecological malignancies involving the abdominal wall are challenging. Curative and palliative approaches often involve substantial surgical resection, which can be technically demanding. The outcomes are poorly understood. This article describes our experiences with the abdominal reconstruction following surgical resection of gynecological abdominal wall malignancy using pedicled anterolateral thigh flap.
Methods: From 2011 to 2012, three patients with gynecological abdominal wall malignancies underwent surgical intervention. One patient had endometrial cancer with a port-site metastasis; two patients had a malignant transformation of abdominal wall endometriosis. All patients presented with a painful mass with or without ulceration. The mean age was 52 years (range 41 to 59). Radical resection resulted in a full-thickness abdominal wall defect for all patients and the reconstruction was accomplished by autologous tissue transfer (i.e., the pedicled anterolateral thigh flap).
Results: Adequate resection margins were obtained and the postoperative course was unremarkable, without flap-related complications in all cases. At a mean follow-up of 24.7 months (range 22 to 30), all patients survived without an evidence of local recurrence. However, detection of lymph node metastasis was identified in one patient. Stable wound coverage, pain relief, and abdominal wall reconstruction were achieved in all patients. No hernias were identified, and all patients were able to walk normally.
Conclusion: Patients with gynecological abdominal wall malignancies can benefit significantly from radical resection and autologous reconstruction. The pedicled anterolateral thigh flap is the preferred donor site, offering a reliable solution to abdominal wall reconstruction in this setting. The satisfactory results should prompt a more aggressive surgical approach for these patients.
Abdominal wall involvement is an uncommon feature of gynecological malignancy. This may represent direct tumour invasion, tumour metastasis to a port-site following surgical resection, or malignant transformation of abdominal wall endometriosis.
Port-Site Metastasis
Laparoscopic techniques are widely used in the staging and treatment of a variety of gynecological cancers. Port-site Metastasis (PSM) refers to an uncommon, yet well-described, phenomenon of tumour recurrence at the sites of trocar insertion. PSM is the leading cause of gynecological abdominal wall malignancy. The incidence varies with different primary tumour sites and increases with the number of previous laparoscopic procedures. It can occur with tumours of both low malignant potential and, more commonly, with high-grade malignancy [1]. Port-site metastasis occurs in as many as 1-2% of laparoscopically investigated/treated ovarian cancers and as few as 0.18-0.33% of endometrial cancers [2,3].
The etiology of PSM is unclear and there are multiple proposed theories in the literature. Surgical factors such as tissue and tumour instrumentation and pressures of insufflated gas causing aerosolization of tumour cells may be associated with the development of port-site metastases. There is also evidence to suggest that the immunological factors at the port-site wound and tumour characteristics may play a role in the development of port-site metastases [1]. It is likely that the process is multifactorial in nature.
PSMs are associated with local recurrence or distant metastases in up to 97% of cases [1]. Isolated PSMs are much rarer, however, hold more potential for curative treatment. Outcomes for these patients are not well understood, largely owing to low numbers of reported cases. Both the isolated and non-isolated PSM hold a poor prognosis. Palomba et al found that only 1 out of 4 cases of isolated PSM was alive and free of disease after 10 months from radiological detection of recurrence [4].
Endometriosis
Endometriosis is defined as the presence of endometrial glands and stroma outside the endometrial lining and uterine musculature. Rarely (<1% lesions) this tissue can undergo malignant transformation, with the ovary being the primary site in 79% of cases [5]. Endometriosis affecting the abdominal wall is typically found near surgical incision sites such as C-section scars, which suggests iatrogenic transplantation of endometrial tissue rather than a process of metastasis [6]. Whilst there are 22 cases of abdominal wall malignancies arising from endometriosis reported in the literature, the condition is relatively uncommon and hence is not well understood. The risk factors for malignant transformation of endometriosis include long-standing endometriosis, endometriosis diagnosed at an early age, infertility and/or a history of infertility treatment, and the presence of ovarian endometriomas [7].
The process of carcinogenesis is likely multifactorial and may be related to oxidative stress from accumulation of free iron and heme from recurrent hemorrhage within endometriotic lesions. Localised inflammation and a hormonal micro-environment of estrogen excess within the endometriotic tissue may also promote premalignant change [7]. Common histological patterns reported in the literature include clear cell carcinoma and endometrioid adenocarcinoma. Less commonly, sarcoma, papillary serous carcinoma, and cystadenocarcinoma are seen.
Direct Tumour Invasion
Gynecological malignancy, which is locally invasive to the extent of involving the abdominal wall, is often irresectable and associated with carcinomatosis. There are no articles in the literature describing successfully resected tumour in these instances.
Treatment Options
Surgical resection may be required as a curative or palliative treatment approach with or without adjuvant or neoadjuvant chemo-radiotherapy. It is critical to obtain a clear and adequate resection margin to avoid local recurrence and hence radical resection is recommended, with a resulting full-thickness abdominal wall defect. For reconstructive purposes, this can be divided into a musculofascial and skin and soft-tissue component, both of which have individual reconstructive needs.
Musculo-fascial defects are commonly reconstructed with a polypropylene mesh if there are adequate skin and soft tissue coverage; however, mesh repairs are associated with a higher incidence of infection, poor wound union, or even mesh extrusion [8,9]. Mesh infection is a serious complication almost always requiring re-operation for mesh removal. In addition, neo-adjuvant and adjuvant radiotherapy can make a mesh-reconstructed abdominal wall more vulnerable to complications.
For large skin and soft-tissue abdominal defects, attempts at primary closure may simply be unfeasible due to the size of the defect involved or be closed under undue tension, resulting in dehiscence [8,10]. Risk factors of malignancy and planned radiotherapy also contribute to poor wound healing and failure of direct closure. Use of autologous extra-abdominal tissue is, therefore, a favourable reconstructive option. Pedicled flaps from the thigh are the primary source of autologous tissue for abdominal wall reconstruction [8,9]. Workhorse flaps include the rectus femoris flap, the tensor fascia lata flap, and the anterolateral thigh flap (ALT) flap.
In the past, the rectus femoris flap was widely used [9]; however, harvesting the rectus femoris muscle can lead to a significant loss of strength for knee extension, resulting in ambulatory difficulty. Thus, fewer surgeons choose the rectus femoris flap. Mathes suggests the use of a flap in full-thickness abdominal wall defects, and the pedicled tensor fascia lata flap is the primary option for most of his cases [8]. However, pre-expansion to recruit more skin territory is required in some cases, and partial flap loss can occur. Williams reported their experiences with abdominal wall reconstruction using either free or pedicled tensor fascia lata flaps and found that partial flap loss occurred in 6 of 15 cases [10]. With this high incidence of partial flap loss, the tensor fascia lata flap may not provide a stable wound coverage and hence not be considered a reliable reconstructive option.
The ALT flap, in contrast, is associated with a more reliable and larger skin paddle and lower donor-site morbidity. Different tissue components, including skin, fascia lata, and vastus lateralis muscle can be harvested. Our unit, Chang Gung Memorial Hospital, has highlighted the reliability and versatility of free ALT flaps [10-12]. When the flap is transferred in a pedicled fashion, the vascular pedicle is long and allows for a wide arc of rotation, making it highly adaptable to soft-tissue coverage for regional defects, including defects in the perineum, groin, abdomen, greater trochanter, and ischium. The vascularized fascia lata component incorporated into the flap can also help to rebuild the musculofascial integrity in abdominal wall defects. In cases of abdominal wall reconstruction using pedicled ALT flaps, high success rates with no hernia had been reported, even if polypropylene mesh was not used [14-24]. According to the literature and based on our experiences, the ALT flap is superior to the tensor fascia lata flap because of its longer vascular pedicle and a more reliable distal skin paddle.
In this article, we evaluated three consecutive cases of surgically treated abdominal wall malignancies of gynecological origin. In all patients, the surgical intervention consisted of laparoscopic exploration followed by radical resection and abdominal wall reconstruction with a pedicled ALT flap. We identified the types of malignancy, patterns of presentation, location and extent of the abdominal wall defects, techniques and outcomes of the reconstruction, adjuvant therapy, and disease status at the long-term follow-up.
From November 2011 through June 2012, three cases of surgically treated gynecologic abdominal wall malignancies underwent radical resection and abdominal wall reconstruction with the pedicled ALT flap.
One patient had previously treated endometrial cancer with a port-site metastasis; two patients had a malignant transformation of abdominal wall endometriosis. Neoadjuvant chemoradiotherapy was administered in one patient in an attempt to downsize a large tumour. Patients’ ages ranged between 41 and 59 years (mean = 52 years).
Surgical Techniques
Laparoscopic exploration was initially performed to identify any intraperitoneal lesions or carcinomatosis not highlighted on preoperative imaging. Once an isolated disease was confirmed, the patient progressed to radical resection of the abdominal wall. Intraoperative specimens were sent for freshly frozen section histology to ensure that the resection margins were clear. An inguinal lymph node dissection was performed if there was a clinical or radiological suspicion of lymph node involvement or if the pre-operative multidisciplinary team discussion recommended prophylactic clearance.
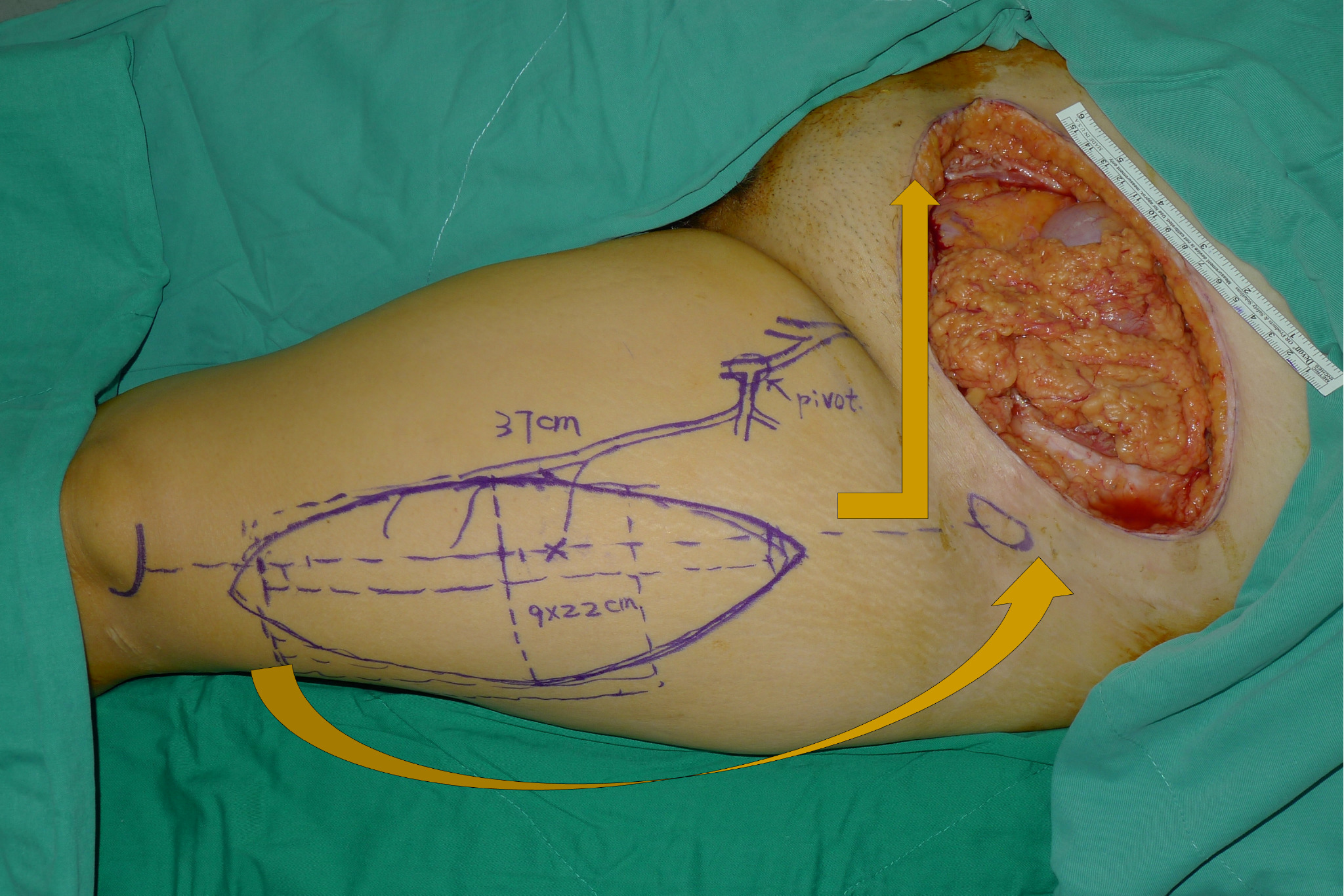
Following resection, the resulting defect was measured, and thigh laterality was chosen for flap-harvest. Dissection in the subcutaneous plane was made from the abdominal wall defect to the inguinal ligament of the chosen thigh. A line was then drawn from the anterior superior iliac spine to the superolateral portion of the patella (A-P line). This line demarcated the septum between the rectus femoris and the vastus lateralis. The lateral circumflex femoral artery arises from the deep femoral artery and gives off a descending branch, which is the main pedicle of the ALT flap. The descending branch sends vascular branches to the vastus lateralis muscle and the overlying skin during its course through the septum. In designing the flap, the medial margin was set at 2-3 cm medial to the A-P line. The flap length and width were determined according to the defect dimensions (Figure 1). The flap harvest began with a medial skin incision and the deep fascia of the thigh was opened. The descending branch was identified in the septum and traced proximally under the rectus femoris muscle to reach the main trunk of the lateral circumflex femoral artery. The sub-muscular tunnel (under rectus femoris) was further advanced to meet the previously dissected subcutaneous tunnel. After the two tunnels were connected, a lateral flap skin incision was made, and the flap was freed from the underlying structures and elevated. The flap was then transferred to the defect through the tunnel (Figure 2A). The vascular pedicle was freed from the adjacent structures, with care being taken to avoid twisting or kinking. The flap inset and the abdominal wall reconstruction were accomplished with a three-layer closure. 1.0 Vicryl sutures were placed between the abdominal musculofascial edge and the flap musculofascial edge (Figure 2B). Interrupted sutures were used to obtain tight musculofascial closure (Figure 2C). Care was taken to avoid the urinary bladder because of its close proximity to the inferior edge of the abdominal musculofascial defect. The second layer was constructed by suturing the Scarpa’s fascia of the abdomen to the superficial fascia of the ALT flap. Skin closure, the third layer, was performed after a drain was placed (Figure 2D). If possible, the donor site was closed primarily; otherwise, a split-thickness skin graft was used.
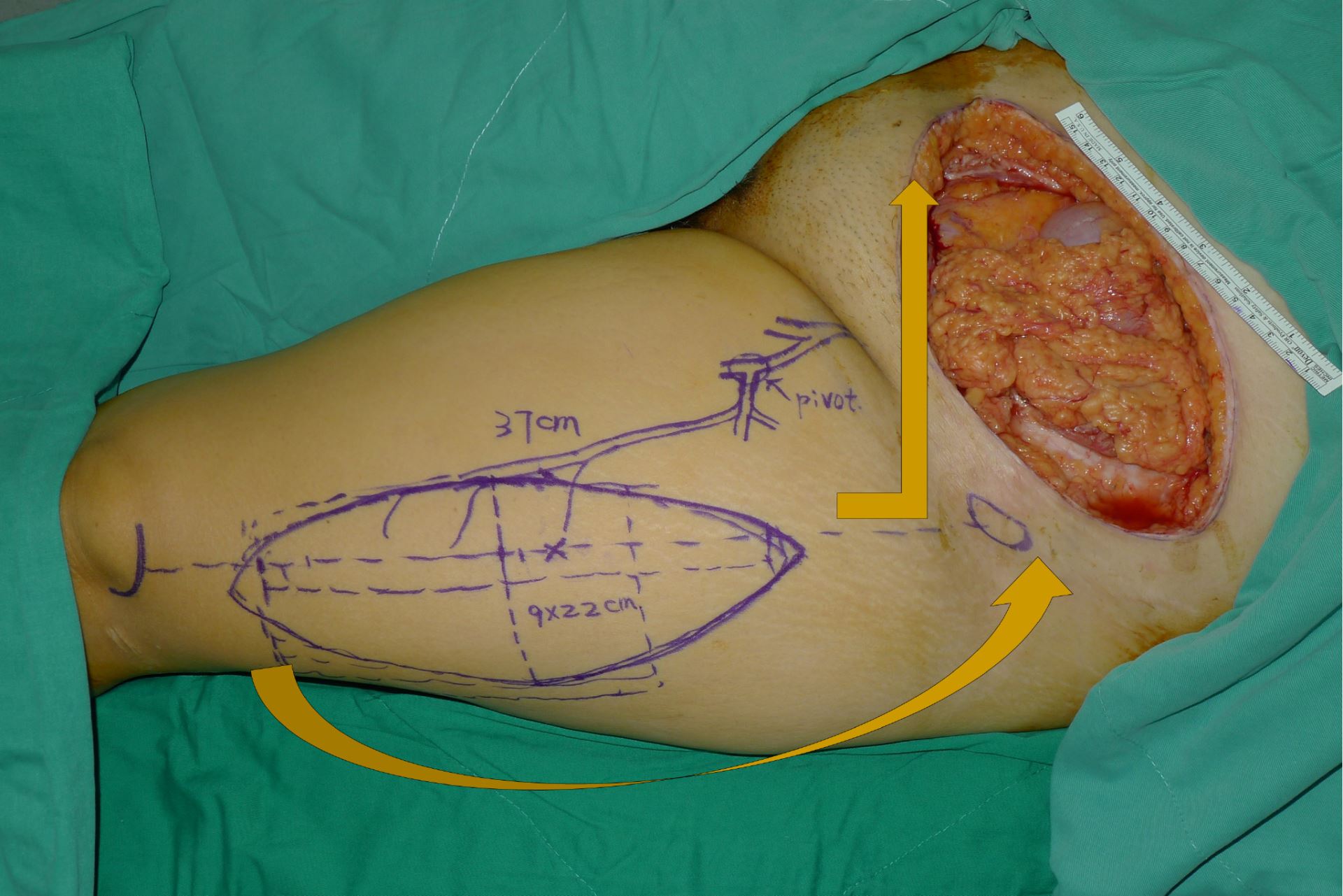
Figure 1. Design of a left-pedicled anterolateral thigh flap for a left lower abdominal wall defect. Arrows denote a counterclockwise flap rotation for a left thigh flap. The rotation becomes clockwise when the right thigh is chosen as the donor site.
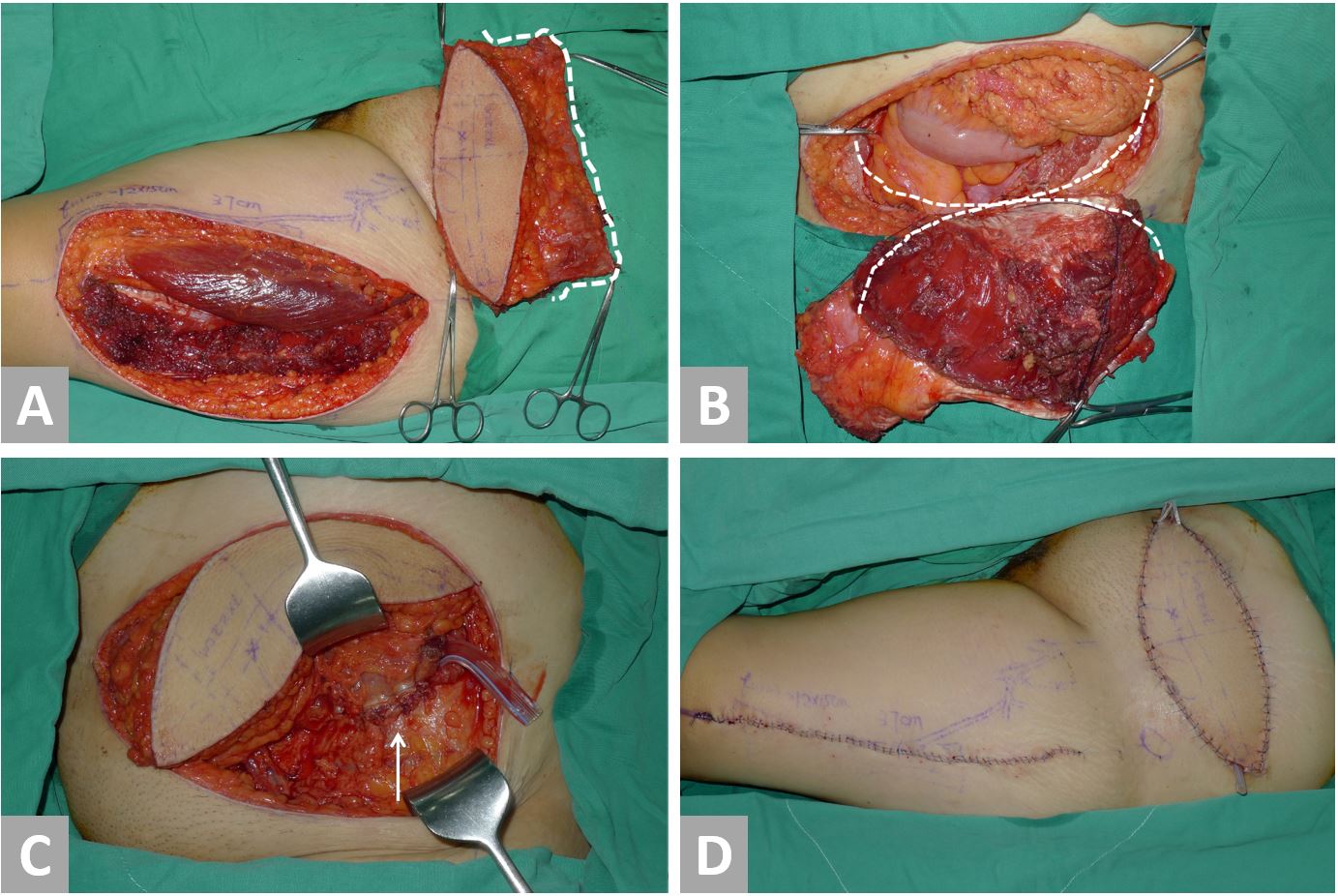
Figure 2. Surgical techniques using Case 1 as an example. (A) The flap is elevated and transferred to the abdomen. Note the vascularized fascia lata component of the flap (white dotted line). (B) The flap inset begins with No-1 Vicryl sutures between the flap fascia lata edge (white dotted line) and the abdominal musculofascial edge (black dotted line). (C) Tight fascial closure is obtained. (D) Completion of abdominal wall reconstruction and donor site closure.
Adequate resection margins were achieved in all patients, resulting in full-thickness abdominal wall defects. In all cases, the defect involved the lower abdomen: one left, one right, and one involving both sides. The musculofascial defect area ranged between 140 to 320 cm2 (mean = 203 cm2), and the skin and soft-tissue defect was slightly larger, ranging between 160 to 375 cm2 (mean = 238 cm2). The flap donor sites were closed primarily in the two unilateral defect cases. The donor site of the patient with a complete lower abdominal defect underwent split-thickness skin grafting.
Postoperatively, the flaps were monitored in a microsurgical intensive care unit for a week as per the protocol for patients undergoing free flap reconstruction in our unit. The patients were then transferred to a regular plastic surgery ward and were later discharged. Hospital stay ranged from 14 to 36 days (mean = 21 days).
Table 1 shows the characteristics of the patients. In Case 1, metastatic iliac lymph nodes were discovered 11 months postoperatively and were managed with laparoscopic pelvic lymph node dissection followed by chemoradiotherapy. Case 2 (Figure 3) was known to have aorta-caval lymph node metastases prior to surgery. These regressed significantly with chemoradiotherapy. Case 3 (Figure 4) remained disease-free. Neoadjuvant chemoradiotherapy was administered in Case 3. The other cases were considered candidates for adjuvant chemo and radiotherapy; however, this was not initially accepted by either patient.
The mean follow-up was 24.7 months (range 22 to 30 months). In this period, there were no flap-related complications such as wound infection, dehiscence, re-operation, partial flap-loss, or flap failure. One patient experienced donor site morbidity, however. During the follow-up period, none of the patients had a local recurrence. Stable wound coverage, pain relief, and successful abdominal wall reconstruction were achieved in all patients. No patients had an evidence of distant (non-lymphatic) metastatic disease during the follow-up. No abdominal wall hernias developed and all patients were able to walk normally and perform most of the daily activities independently.
Case 1
This patient was a 41-year-old female without a significant past medical history (Figure 2, No. 1 in Table 1). Twelve years prior to admission, she developed a mass on the left side of her cesarean section scar from a delivery one year before. The pain was cyclical in relation to her menstrual cycle. In light of rapid enlargement from the mass and increasing pain, she underwent an incisional biopsy demonstrating endometrioid adenocarcinoma arising from a background of endometriotic tissue. Preoperative computed tomography (CT) imaging excluded any evidence of distant metastases; however, clinically and radiologically, a lymph node in the left inguinal region had appearances suspicious for metastatic disease. The patient underwent radical resection with a resulting defect size of 9 x 20 cm of skin and soft tissue and 10 x 15 cm of fascia. The defect was reconstructed with a myocutaneous ALT flap with vastus lateralis muscle of dimensions 9 x 22 cm. She underwent bilateral inguinal lymph node dissections at the same time. The donor site was closed directly. There were no postoperative complications and she was discharged afterward. Histopathology confirmed adenocarcinoma of ectopic endometrial tissues that had been completely excised.
Although the patient was offered adjuvant chemoradiotherapy, she initially declined. In the 11 months postoperative period, however, a routine CT scan revealed pelvic lymph nodes suspicious for metastasic disease and she underwent laparoscopic pelvic lymph node dissection and chemoradiotherapy. Thirty months postoperatively, she was completely pain-free (pre-op pain score 6) and she was able to perform all activities of daily living unaided. There was no further evidence of carcinomatosis on subsequent CT imaging.
Case 2
Case 2 was a 56-year-old female with a history of FIGO stage IIIC endometrial cancer (Figure 3, No. 2 in Table 1). This patient was treated with laparoscopic staging surgery followed by adjuvant chemoradiotherapy. Seventeen months postoperatively, she found an enlarging painful mass at one of the trocar sites in her right lower abdomen. She presented two months later with an ulcerated wound. Computer Assisted Tomography demonstrated a 5-6 cm mass on the right lower abdominal wall with metastatic lymph nodes in the right inguinal and aortocaval regions. A punch biopsy of the mass confirmed adenocarcinoma. Surgical excision was performed, which involved radical resection and inguinal lymph node dissection. The resultant musculofascial defect was 10 x 14 cm2 and the skin defect was 8 x 20 cm2. A right-pedicled ALT flap with vascularized fascia lata was transferred for reconstruction. Layer-by-layer abdominal wound closure was performed and the donor site underwent primary closure. Histopathology revealed metastatic endometrioid adenocarcinoma, which was completely excised. The postoperative course was uneventful and the patient was discharged after 14 days.
Adjuvant radiotherapy was administered followed by six courses of cisplatin-based chemotherapy. During a 10-month follow-up, a CT scan confirmed that the metastatic aorta-caval lymph nodes had regressed significantly and there was no evidence of further spread. At 22-month follow-up, the patient was able to walk normally and could perform most of the daily activities. The patient did not develop a hernia. On a subjective pain scale (with 0 being no discomfort and 10 being intolerable pain), the patient reported a preoperative pain score as 8; at follow-up, the pain score improved to 0.
Case 3
Case 3 was a 59-year-old woman of parity one who presented with a painful lower abdominal wall mass (Figure 4, No. 3 in Table 1). She had undergone a cesarean section at the age of 24 years and shortly after started experiencing cyclical pain near the scar during menstruation. Nine years later, she underwent a total hysterectomy and right salpingo-oophorectomy for treatment of her endometriosis. Following surgery, her cyclical abdominal pain persisted, and a mass was later discovered by the patient adjacent to the C-section scar. This mass enlarged over the next two years and eventually, her pain became persistent. A PET-CT (Positron emission tomography-computed tomography) confirmed a 10-cm lower anterior abdominal wall tumor without an evidence of metastasis. Serum tumor marker levels, including CEA, CA-125, and CA-153, were insignificant. A biopsy revealed adenocarcinoma that was thought to be originated from the ovary. Six courses of cisplatin-based chemotherapy were administered with concurrent radiotherapy. Significant tumor regression was shown on a CT scan, three months after neoadjuvant chemoradiation was completed. Surgical intervention was then undertaken with immediate reconstruction. The surgery involved the resection of the entire lower half of the abdominal wall with omentectomy and a left salpingo-oophorectomy. A right-pedicled ALT flap with vascularized fascia lata and a skin paddle measuring 14 x 27 cm2 was transferred for reconstruction. A sturdy layer-by-layer closure was performed and the donor site was skin grafted.
Macroscopic examination revealed a tumour measuring 9.2 x 6 x 4 cm3. The histological appearances were consistent with a clear cell carcinoma arising from endometriosis and the tumour was completely resected with clear margins. The other tissue submitted for pathological examination did not show any evidence of malignancy. The patient had problems with a poor graft taken over the donor site, which required an extended stay for dressings, and more lengthy rehabilitation gave the extent of the resection and reconstruction. Her postoperative course was otherwise uneventful and the patient was discharged after 36 days. During the 9-month follow-up, a CT scan did not show any evidence of local or distant metastatic disease and the patient did not develop a hernia. At 22-month follow-up, the patient was able to walk normally and could perform most daily activities. The patient reported a decrease in her pain score from 7 pre-operatively to 2 after the follow-up.
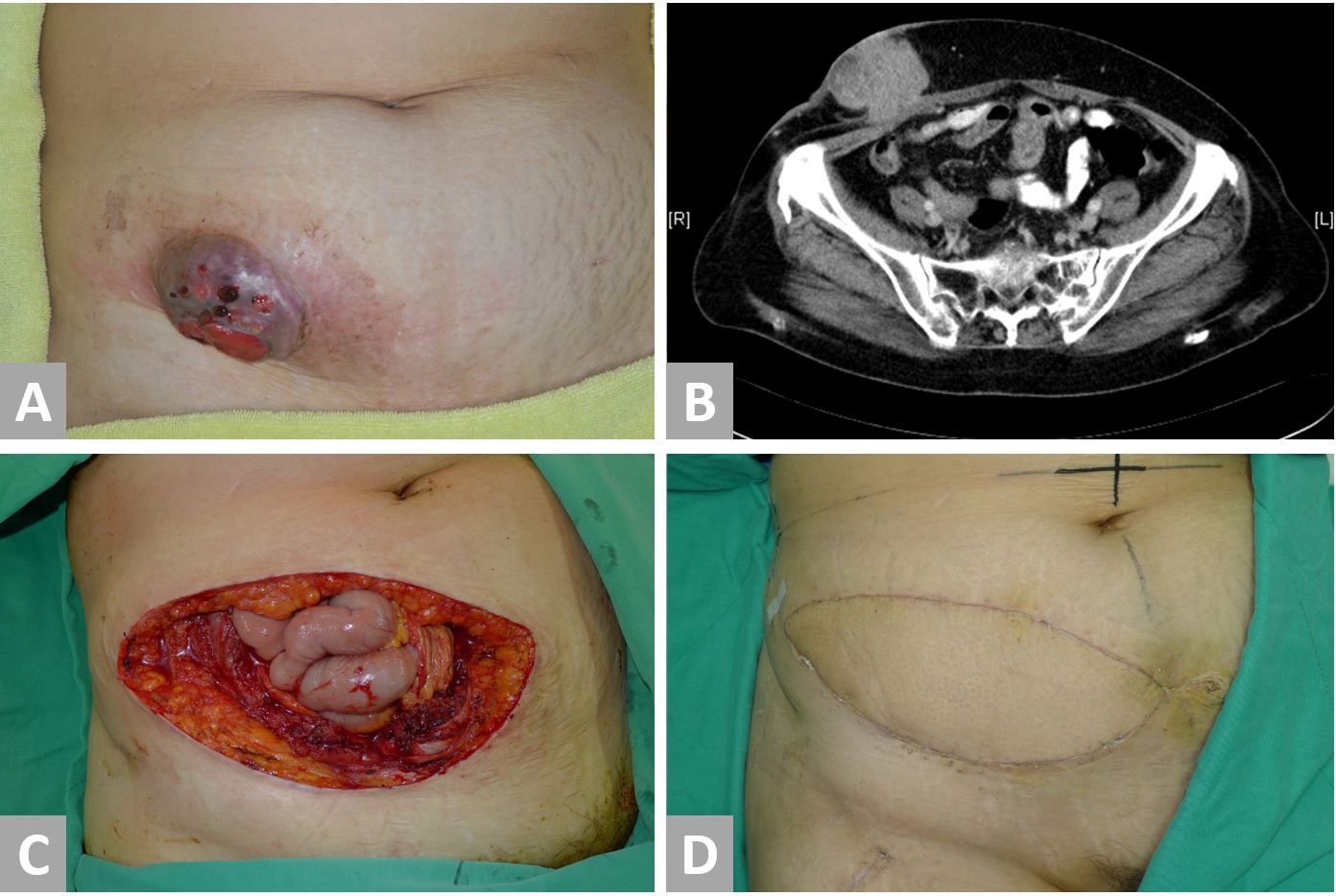
Figure 3. Case 2. A 56-year-old patient with a port-site metastasis of endometrial cancer. (A) Gross appearance of tumor at the right lower abdomen. (B) A CT scan shows a tumor involving the right abdominal skin, fat, fascia, and muscle. (C) Radical resection results in a defect at the right lower abdomen with a skin defect of 8 x 20 cm2 and a musculofascial defect of 10 x 14 cm2. (D) Reconstructed abdomen at six weeks postoperatively.
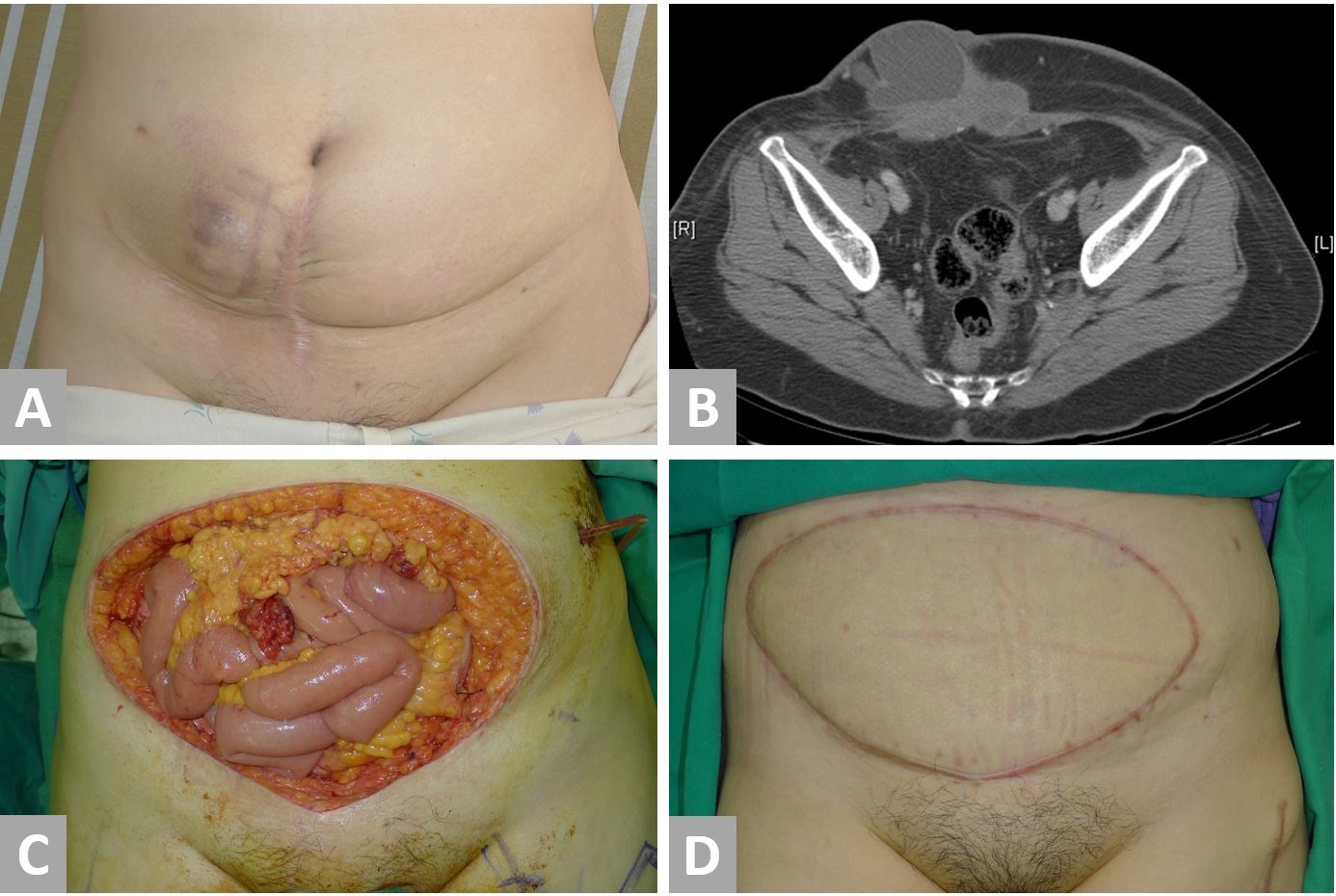
Figure 4. Case 3. A 59-year-old patient with a malignant transformation of abdominal wall endometriosis. (A) Preoperative appearance of the tumor. (B) A CT scan shows a tumor involving abdominal skin, subcutaneous fat, fascia, and muscle. The tumor has a cystic and a fibrous component. (C) Radical resection results in a defect of the entire lower half of the abdomen with a skin defect of 15 x 25 cm2 and a musculofascial defect of 16 x 20 cm2. (D) Reconstructed abdomen at eleven months postoperatively.
Three patients with gynecological abdominal wall malignancies were managed with radical resection followed by immediate reconstruction with a pedicled ALT flap and vascularized fascia lata or vastus lateralis. Radical resection resulted in adequate resection margins in all the patients, which was made feasible by autologous soft tissue reconstruction with the ALT flap. A study addressing the outcomes of the patients with isolated PSMs from endometrial cancer showed 4 out of 6 patients undergoing resection had positive, focally positive, or close (<0.5 mm) margins [28]. It was not clear how wide the intended resection margins were and what reconstructive techniques were used, however. Autologous pedicled or free flaps permit reconstruction of large defects and hence radical resection with wider margins can be obtained. This may reduce the risk of recurrence from insufficient pathological margins; however, Grant et al did not demonstrate a difference in the overall survival between those with positive and negative margins. Interestingly, the patient with the longest survival (disease-free or otherwise) did not undergo any surgical resection.
Zivanovic found that the patients who developed PSMs within 7 months of surgery had a worse prognosis than the patients who developed PSMs beyond 7 months post-surgery [3]. Earlier development of detectable PSMs could, therefore, be suggestive of more aggressive malignancy. In our patient, PSM became clinically detectable 17 months after laparoscopic staging surgery, and hence a radical treatment strategy was adopted.
PSMs are also commonly associated with metastatic regional lymph nodes or carcinomatosis. In our patient, the malignant appearing lymph nodes were detected in the aorto-caval and inguinal regions at the time of diagnosis. Following radical resection with adjuvant chemoradiotherapy, there was no evidence of progressive disease during 10 months post resection follow-up and the patient was still alive at 22 months. Although the resection was unlikely to be curative, it appeared that good quality of life was achieved with a reduction in pain score and a satisfactory level of independence. This suggests that there is a role for wide resection with autologous reconstruction in patients with non-isolated PSM.
Previous reports [4,29] suggest that both the isolated and non-isolated PSMs have poor outcomes, which suggests that "isolated" PSM may actually be associated with the clinically and radiologically undetectable micrometastatic disease [27]. Malignancy arising from endometriosis largely occurs in the ovary; however, extra-ovarian sites of malignancy comprise approximately 25% of cases [31]. Malignancies arising from endometriosis on the abdominal wall are even rarer, with only 22 reported cases [32]. The tumor is often preceded by endometriosis that develops near a C-section scar [32], as in our patients. In the two cases in our series, the histological patterns were clear cell carcinoma and endometrioid adenocarcinoma, which were among the most common histological patterns in malignant transformation of extra-ovarian endometriosis. The interval between the formation of benign endometriosis and its malignant transformation ranges from 3 to 39 years [33]. In our patients, the intervals were 12 and 26 years.
Due to its rarity, it is difficult to establish a standard treatment protocol or to assign a prognosis of malignant transformation of endometriosis. However, surgical resection is the main treatment option and has been adopted in all reported cases to our knowledge. In addition, a follow-up of seven years without a recurrence has been reported [31,34], indicating that an aggressive treatment strategy is preferable whereever appropriate. Whilst there is a lack of evidence to support adjuvant radiotherapy and chemotherapy, there may be a role for neoadjuvant chemotherapy to downsize large tumours in order to reduce the overall surgical defect depending on the histological type [35]. We also found that neither of our patients progressed to carcinomatosis. Although one patient developed lymph node metastases to the deep pelvic lymph nodes in the context of previously cleared inguinal lymph node metastases, this patient had declined adjuvant chemo-radiotherapy initially, which might have reduced the chances of this progressing. It was possible that micrometastatic disease was present in the deep pelvic lymph nodes at the time of diagnosis. It is not known whether both inguinal and pelvic lymph nodes should be cleared if just inguinal lymph node metastases were detected at diagnosis. This perhaps suggests a rationale in aggressive lymph node clearance and/or adjuvant chemoradiotherapy, especially in young patients with chemosensitive histology types without the evidence of distant metastases in improving disease-free and overall survival.
Our case series involved patients who were less than 60 years old with no significant comorbidities such as cardiovascular disease, diabetes, smoking, or steroid use. The aggressive approach for these patients was therefore merited due to a low comorbidity burden, favourable factors for wound healing, and long potential gains in high quality life years. It is unknown whether this approach would be appropriate for more elderly patients or those with significant concurrent diseases. Our follow-up is limited and therefore disease recurrence cannot be entirely excluded.
Reconstruction of abdominal wall defects involves a complex decision-making tree. If stable skin and soft-tissue coverage are present, polypropylene mesh is the primary option used to bridge musculofascial gaps [8,9]. When a full-thickness defect is present, the use of a flap is usually advised [8,9,36]. In our cases, it would have been possible to perform polypropylene mesh repair and primary wound closure with an abdominal advancement flap in two cases (Cases 1 and Case 2). However, polypropylene meshes may lead to higher rates of infection, poor wound union, and mesh extrusion compared to the use of autologous tissue. Primary closure of the abdominal wound may also result in tension, contributing to wound dehiscence [8]. Furthermore, an alloplastic material in a wound that was or is to be irradiated makes the reconstructed area more vulnerable to complications. It has been suggested that the defects in an irradiated area are better managed with flaps originating outside the radiation field [37]. Therefore, autologous reconstruction should be the primary option as it provides well-vascularized tissue with better resistance to radiation and infection.
With proper design and techniques, the pedicled ALT flap can be used to reconstruct extensive abdominal defects as large volumes of skin and soft tissue can be transferred to provide tension-free closure and stable wound coverage. To allow for the furthest reach, the vascular pedicle must sometimes be mildly stretched, thus minor twisting or kinking cannot be tolerated, as this can contribute to venous congestion and compromise the viability of the flap. It is therefore imperative to examine the whole course of the vascular pedicle when transferring the flap. The donor site is closed directly if possible, which may lead to the formation of “dog ears” due to the generous width of the flap in comparison to its length. Where direct closure is not possible split-thickness skin grafting can be performed. Complications include hematoma, seroma, infection, scarring, dehiscence, paresthesia, and scarring.
Patients with gynecological abdominal wall malignancies can benefit significantly from radical resection followed by autologous reconstruction. This can be potentially curative but also lend substantial palliative benefits when disease clearance is unlikely or not possible. Autologous reconstruction is preferred for the following reasons: (1) permission of wider resection margins and therefore more likely curative resection through use of extra-abdominal tissue in reconstruction; (2) provision of sufficient volumes of tissue for reliable wound coverage and stable abdominal wall reconstruction; and (3) providing well-vascularized tissue robust enough to tolerate radiation and reduce radiotherapy-associated local complications. The pedicled ALT flap, with its long pedicle, ease of harvest, strong fascia, reliable and large skin paddle, and a wide arc of rotation in the abdomen, serves as a reliable option in this setting. With these techniques, most gynecological abdominal wall malignancies can be radically resected and the defect can be reliably reconstructed. The satisfactory results in our patients should prompt a more aggressive surgical approach for similar patients.
Received date: April 13, 2017
Accepted date: December 27, 2018
Published date: March 02, 2019
M. Abdelrahman and C.W. Wu contributed equally to this work.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2019 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Pedicled anterolateral thigh flap is a versatile option for reconstruction of complex soft tissue defects in varied anatomical regions. Its wide arc of rotation and less donor site morbidity are its added advantages.
Abdelrahman M, Wu CW, Couves A, Alsharkawy K, Huang KG. Pedicled anterolateral thigh flap for abdominal reconstruction following surgical resection of gynecological abdominal wall malignancy. Int Microsurg J 2019;3(1):3. https://doi.org/10.24983/scitemed.imj.2019.00102