Objective: Constraint-induced music therapy (CIMT) has been shown to enhance hearing recovery in patients with sudden sensorineural hearing loss (SSNHL) by preventing maladaptive reorganization of the auditory cortex. Our objective in this study was to assess the effectiveness of CIMT in restoring hearing among patients with SSNHL.
Methods: The study included prospective (CIMT group) and retrospective (non-CIMT group) study arms. CIMT is characterized by (1) plugging the healthy ear to induce temporary artificial hearing loss (i.e., constraint) and (2) simultaneous acoustic stimulation of the affected ear using relaxing music. The outcome variables used to evaluate hearing recovery included (1) hearing threshold, (2) interaural hearing gap, and (3) hearing recovery rate. We measured the P300 component of long-latency auditory evoked potential to analyze brain activity to determine the appearance of neuroplasticity in assessing a subgroup of six patients with CIMT. All of the patients in the study also received conventional steroid therapy.
Results: The CIMT and non-CIMT groups were comparable in terms of pre-treatment hearing level (P = 0.710), age (P = 0.124), gender (P = 0.272), and side of hearing loss (P = 0.132). In both groups, we observed a significant improvement in hearing thresholds at two weeks and four weeks after treatment (all P <0.01). Nevertheless, we did not observe a statistically significant difference in hearing recovery when using hearing threshold, interaural hearing gap, or hearing recovering rate as outcome variables (P >0.05). This observation was verified using multivariate analysis (non-CIMT vs. CIMT, odds ratio 3.84, 95% confidence interval 0.18-81.65, P = 0.388 at two weeks after treatment; odds ratio 2.70, 95% confidence interval 0.15-47.60, P = 0.497 at four weeks after treatment). P300 measurements conducted on affected ears failed to identify significant signs of neuroplastic change in response to CIMT (P = 0.063 for the amplitude comparison; P = 0.094 for the latency comparison).
Conclusion: CIMT is a safe, cost-effective addition to corticosteroid treatment for SSNHL patients. It is also possible that CIMT provides an enjoyable therapeutic adjunct for the relief of stress and anxiety associated with SSNHL. However, our results failed to identify the additive effect of CIMT on hearing recovery in patients with SSNHL. Our study was also unable to confirm the degree of neuroplasticity in patients with CIMT.
Sudden sensorineural hearing loss (SSNHL) refers to a subjective sensation of hearing impairment over a period of less than 72 hours. This condition is typified by specific audiometric criteria: (1) a decrease in hearing of ≥30 decibels (dB), compared to threshold of the premorbid or opposite ear (if premorbid audiometry is unavailable); and (2) hearing loss that affects at least three consecutive frequencies [1]. SSNHL is an otologic emergency, which can be a highly distressing experience for patients, and particularly for those who depend on their hearing for work (e.g., musicians, professional drivers, or athletes). SSNHL can have a tremendous impact on one’s quality of life and is often indicative of an elevated risk of adverse cognitive and functional outcomes. In the United States, the number of new cases each year has been estimated at 4,000 [2]. A population-based cross-sectional investigation of SSNHL epidemiology in Germany revealed an incidence of 160 cases of SSNHL per year per 100,000 inhabitants [3]. The high incidence of SSNHL is reflected in the fact that the World Health Organization and the European Union do not consider it a rare disease (prevalence less than 50 per 100,000 inhabitants) [3]. SSNHL typically occurs in middle adulthood and the incidence increases with age [4-6]. Males and females are equally affected [4].
SSNHL can be attributed to an abnormality of the auditory nerve or higher aspects of central auditory perception or processing [1]; however, the cochlea is generally considered the most probable lesion site [7]. The fact is that only 10% of SSNHL cases can be identified, the most pressing of which are acoustic neuroma, stroke, and malignancy [8]. The remaining 90% are idiopathic, presumptively attributed to vascular, infectious, immunologic, or multiple etiologies [1]. This lack of clarity regarding etiology has led to the development of various therapy modalities, including systemic and intratympanic steroids, antiviral agents, anticoagulants, volume expanders, vasoactive substances, antioxidants, hyperbaric oxygen, anti-anxiety medication, diuretics (alone or in combination) [1], or observation alone [9]. Among the various treatment modalities, a tapering course of steroids has been widely adopted as the principal treatment for idiopathic SSNHL, with a reported success rate of 50 to 80% [4,10,11]. Nevertheless, several systematic reviews of randomized controlled trials have failed to determine the role of corticosteroid treatment for SSNHL, with conflicting outcomes [1,12-16]. Furthermore, many patients are not candidates for these substances, due to potentially severe side effects, including the suppression of hypothalamic-pituitary-adrenal function, insomnia, weight gain, gastritis, mood changes, hyperglycemia, hypertension, cataracts, opportunistic infections, osteoporosis, and osteonecrosis [1,17]. There is a lack of adequately powered randomized trials to support the clinical benefits of other treatment options [1].
Thus, the focus of research on SSNHL has shifted toward neuroplasticity-targeted intervention [18]. Neuroplasticity (i.e., neural plasticity) refers to the ability of the nervous system to reorganize its structure, function, and connections in response to environmental stimuli or demands [19]. It was not until the late 1960s that Raisman introduced the term "neuronal plasticity" to describe a permanent change of the neuropil in the septal nuclei of adult rats in response to original deafferentation. Decades of research have now shown that the brain is capable of responding dynamically to a variety of internal and external stimuli [20]. Recent studies have also indicated that neuroplasticity is a fundamental lifelong property of the nervous system [19,21]. In a recent study by Okamoto et al. [22], constraint-induced sound therapy was used to treat patients with idiopathic SSNHL to eliminate or reduce the effects of maladaptive cortical reorganization. The biological plausibility of this approach is based on a neuro-rehabilitation approach and a large body of animal experiments [23-27]. The concept of constraint-induced therapy has previously been used in stroke rehabilitation [28-34]. This involved having hemiplegic patients use their affected limbs while preventing them from using the healthy counterpart by imposing physical constraints. Okamoto et al. also applied this approach to the neuro-rehabilitation of patients with SSNHL. They reported that patients who received constraint-induced sound therapy in conjunction with standard corticosteroid therapy enjoyed significantly better hearing recovery than did those who received only corticosteroid treatments. They attributed those results to brain activity preventing maladaptive reorganization of the auditory cortex.
Nevertheless, there is a lack of clinical evidence to support assertions pertaining to the neurophysiological implications of neuroprotective acoustic training in patients with idiopathic SSNHL. To the best of our knowledge, this was only the second study to provide scientific evidence related to the neuroplasticity of the central auditory nervous system in response to constraint-induced acoustic rehabilitation. Our objective in this study was to assess (1) the additive effect of neuroplasticity on hearing recovery and (2) the unique capacity of constraint-induced acoustic stimulation to prevent maladaptive cortical plasticity in patients with idiopathic SSNHL.
Ethical Considerations
One arm of this study involved the prospective collection of data between March 1, 2018, and November 1, 2018 (8 months), during which subjects received conventional steroid treatment as well as music therapy. We also conducted a retrospective collection of data for the period between April 1, 2016, and August 1, 2018 (2 years), during which subjects received only conventional steroid therapy. The study was conducted in accordance with the Declaration of Helsinki. The prospective study protocol was approved by the Institutional Review Board of Tri-Service General Hospital (No. 1-106-05-189). The purpose, procedures, potential risks, and benefits of the study were thoroughly explained to all candidates, and written informed consent was obtained from all patients prior to participation.
Subjects
Inclusion criteria
We specified predefined inclusion and exclusion criteria for participation in the study to minimize potential complications associated with interpretation. Eligible patients were recruited from the Department of Otolaryngology-Head and Neck Surgery, Taoyuan Armed Forces General Hospital, Taiwan.
Enrollment of eligible patients was based on a diagnosis using the following predefined inclusion criteria: (1) unilateral idiopathic sensorineural hearing loss of ≥30 dB, which is related to the threshold of the premorbid or opposite ear (in cases where premorbid audiometry was unavailable, we assumed that the audiogram of the unaffected ear is similar to the pre-SSNHL audiogram of the affected ear); (2) sensorineural hearing loss affecting at least three consecutive frequencies; and (3) ≤5 days since symptom onset [22].
Exclusion criteria
Participants were excluded if they met any one of the following criteria: (1) age <20 and >70 years; (2) previous or family history of SSNHL; (3) neurological, psychiatric, or cognitive deficits; (4) history of head trauma; (5) air-bone gap of the affected ear, regardless of whether it was associated with a disorder of the middle ear or outer ear; (6) absolute or relative contraindication for steroids, such as a documented allergy to any steroid [35], diabetes with poor control [36,37], hypertension with poor control [37], gastrointestinal ulcerations [37], and a tendency for acne [38]; (7) we also excluded patients who refused to undergo music therapy in the prospective study arm; and (8) those with retro-cochlear causes for SSNHL as evidenced by abnormal differences in interaural latency in wave V of auditory brainstem responses (ABRs) and magnetic resonance imaging of the brain [39].
Treatment Protocols
Steroid therapy
All of the eligible patients (in both study arms) were admitted to the hospital to receive a course of corticosteroids over a period of 7 days (1 mg/kg/day), the dosage of which was subsequently tapered over a period of 7 days (0.5 mg/kg/day for 4 days and 0.25 mg/kg/day for 3 days).
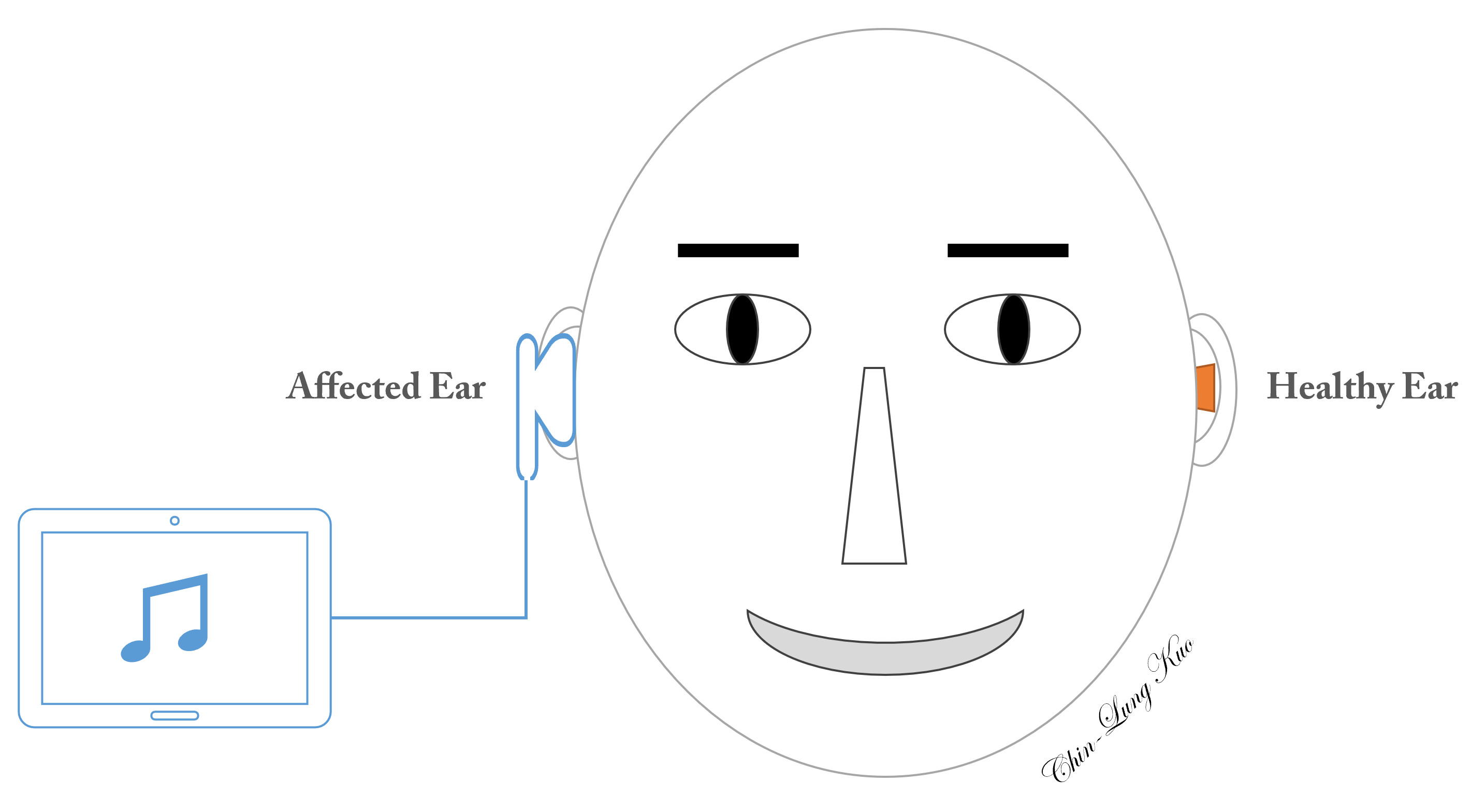
Constraint-induced music therapy (Figure 1)
In the prospective study arm, constraint-induced music therapy (CIMT) included two major components: (1) plugging the intact ear with a foam ear plug to avoid mechanical acoustic input to the cochlea of the healthy ear, thereby constraining afferent electrical neural impulses on the contralateral auditory cortex; and (2) the simultaneous stimulation of the affected ear in an attempt to prevent maladaptive auditory cortical plasticity on the healthy side [22]. The equipment used to administer CIMT included a music player installed on a tablet (Asus ZenPad 10 Z301 Series, ASUSTeK Computer Inc., Taiwan), a pair of headphones (E-books S39 Gaming Volume Control Hook Headset, Chung Ching Technical Co., Ltd, Taichung, Taiwan), and a foam ear plug (3M 1100, 3M Co., Minnesota, USA). To enhance the motivation of patients to engage in the music program, we applied relaxing music rather than pure tones or noise [40]. Patients eligible for CIMT underwent a daily six-hour music program (2 hours in the morning, afternoon, and evening), every day for a period of four weeks. Patients adjusted the volume in one of two ways: (1) to a level at which the music sounded as similar as possible to the sounds before SSNHL [22], or (2) to a level that felt most comfortable to the healthy ear. The sound level was limited to 80 dB [41]. Volume adjustment was meant to increase sound levels in the range of frequencies affected by hearing loss and to avoid potential complications associated with loudness.
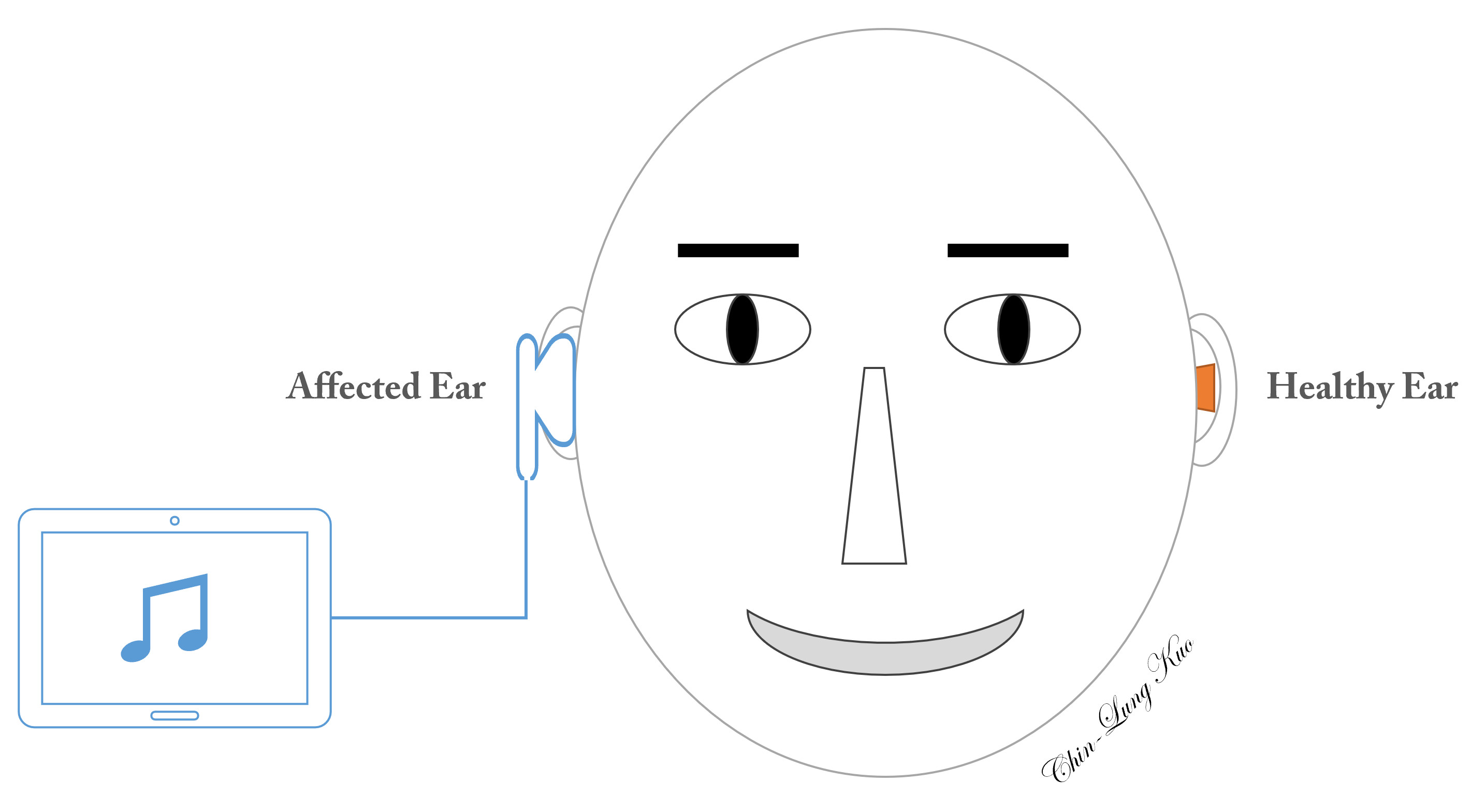
Figure 1. Schematic illustration of constraint-induced music therapy. Music is presented to the affected ear, while the ear canal of the unaffected side is plugged to prevent the admission of sound waves.
Measurements
Audiometric examinations
All participants underwent pure tone audiometry (PTA) examinations using an audiometer (Audiometer GSI-68, Grason-Stadler Inc., Minnesota, USA) to determine the air and bone conduction thresholds of affected and unaffected ears, using a step size of 5 dB at frequencies between 250 Hz and 8000 Hz, in accordance with the modified Hughson-Westlake procedure [42]. Data were compiled on pre- and post-treatment PTA with mean thresholds determined at 0.5, 1, 2, and 3 kHz. The hearing thresholds of both ears were measured every day during the hospital stay to monitor recovery. Hearing thresholds of affected and unaffected ears were also measured when the patients came to our outpatient department for follow-up one week and three weeks after discharge.
Neuroplasticity measurements: auditory evoked potential (P300)
The P300 component of long-latency auditory evoked potential was captured using an evoked potential system (Intelligent Hearing Systems Smart-EP, Intelligent Hearing Systems Co., Florida, USA) for the measurement of neuroplasticity. The skin was cleaned with abrasive paste before attaching electrodes to the skin with electrolytic paste and adhesive tape. P300 was recorded using the oddball paradigm, in which two tone burst stimuli were presented in a random order. One of the two stimuli (standard stimulus, 1000 Hz) was presented more than the other (deviant stimulus, 2000 Hz). These monaural stimuli were delivered to the affected or the healthy ear for 70 ms at 80-90 dBnHL (standard 90 dBnHL and target 80 dBnHL) at a presentation speed of 1.1 stimuli per second using an exact Blackman envelope. The participants were instructed to count the deviant stimulus, which was presented with probability of 15%. P300 was recorded using four electrodes placed at Fz (active electrode), Fpz (ground electrode), and M1 and M2 (mastoids, reference electrodes). The total number of stimuli ranged between 450 and 550. Responses were collected using impedance values below 5 kohms with band-pass filters of 1 to 30 Hz.
Data Analysis and Statistics
For both the CIMT and non-CIMT groups, differences in mean hearing threshold were measured in both ears across all measured frequencies during all examinations; i.e., at the time of admission and at two weeks and four weeks after admission. Paired samples t-tests were used to determine the differences between pre- and post-treatment hearing thresholds in order to assess the degree of hearing recovery.
The variables used to evaluate hearing recovery included the following: (1) hearing threshold; (2) interaural hearing gap (i.e., difference in mean hearing threshold between ears) [22]; and (3) hearing recovering rate = (pre-treatment air conduction hearing threshold of the affected ear – post-treatment air conduction hearing threshold of the affected ear) / (pre-treatment air conduction hearing threshold of the affected ear – air conduction hearing threshold of the intact ear), under the assumption that the audiogram of the intact ear was similar to the audiogram of the affected ear prior to the occurrence of SSNHL [22,41].
A Pearson's chi-square test or Fisher's exact test was used when analyzing categorical variables. Multivariate logistic regression was used to identify the factors that affect hearing recovery. Complete hearing recovery was defined as a hearing threshold of ≤25 dB (normal hearing). Factors used as predictors of hearing recovery included age, gender, side of ear, pre-treatment hearing level, and administration of CIMT.
P300 analysis for the CIMT group was based on the presence/absence of responses for each ear. We calculated the mean latencies (millisecond, ms) and amplitudes (microvolt, μV) in each ear before treatment and four weeks after treatment. A paired sample t-test was used to determine the difference between pre- and post-treatment mean latencies (ms) and amplitudes (μV) in both ears in order to evaluate the degree of neuroplasticity.
All statistical analysis was performed using the commercially available software package SPSS, version 18.0 (SPSS, Inc., Chicago, IL, USA), and p values of less than 0.05 were considered statistically significant.
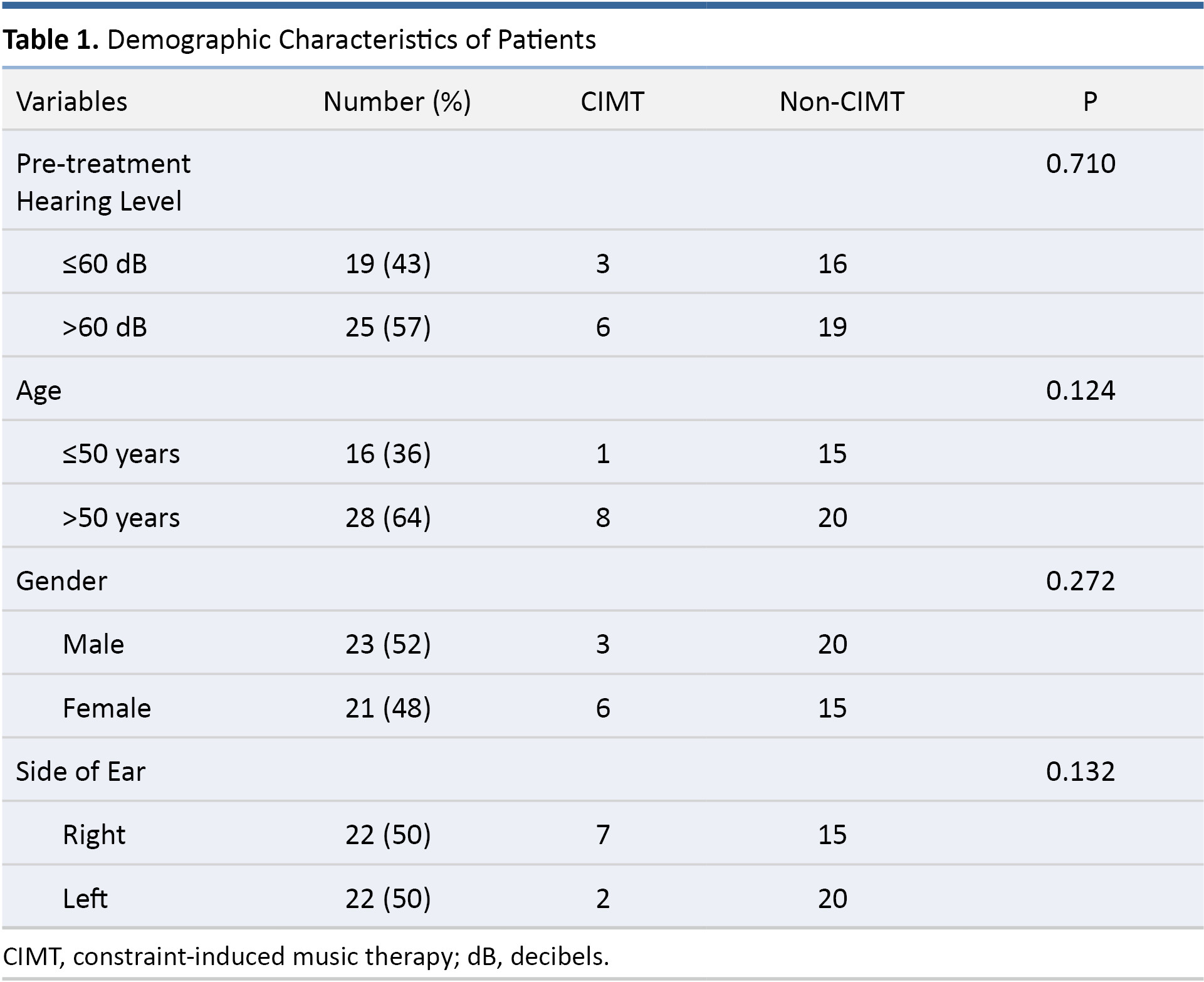
Patient characteristics are listed in Table 1. Among the 44 patients included in the study, 9 patients were included in the prospective CIMT study arm and 35 patients in the retrospective non-CIMT study arm. The 9 patients in the prospective CIMT group included 6 females/3 males, involving 7 right ears and 2 left ears. The mean age of CIMT patients was 57.44 years (P = 0.346). None of the patients who received CIMT presented complications or side effects, and all of the CIMT participants described it as an enjoyable therapeutic addition that helped relieve the stress and anxiety associated with SSNHL. The 35 patients in the non-CIMT retrospective group included 15 females/20 males involving 15 right ears and 20 left ears. The mean age of non-CIMT patients was 51.23 years (P = 0.346).

We selected 60 dB as the cutoff pre-treatment hearing level, because the mean hearing threshold of all participants prior to treatment was 63.68 dB. An age threshold of 50 was adopted for the classification of patients as older or younger, because the mean age of the participants was 52.5 years. As shown in Table 1, the CIMT and non-CIMT groups were comparable in terms of pre-treatment hearing level (P = 0.710), age (P = 0.124), gender (P = 0.272), and side of ear (P = 0.132).
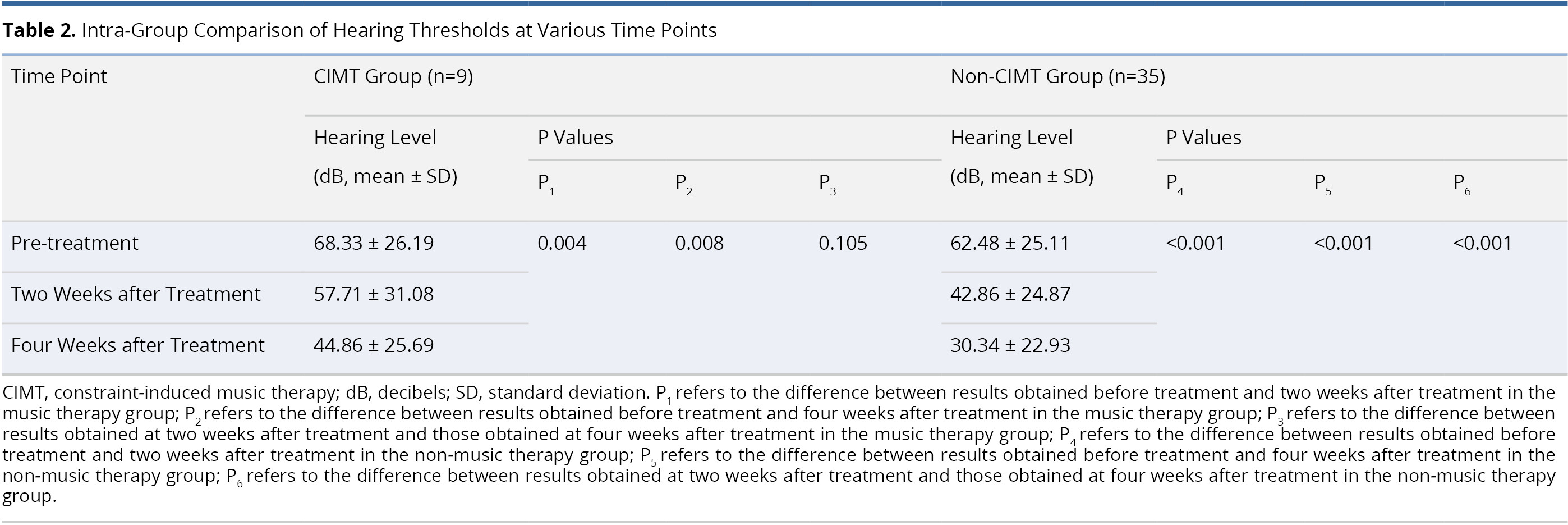
Table 2 lists an intra-group comparison of audiometric results from patients in the CIMT and non-CIMT groups. In the CIMT group, the mean post-treatment hearing threshold at two weeks post-treatment (57.71 ± 31.08 dB) and four weeks post-treatment (44.86 ± 25.69 dB) was significantly better than before treatment (68.33 ± 26.19 dB; P = 0.004 and 0.008, respectively). However, we did not observe a significant difference between the mean hearing thresholds at two weeks and four weeks post-treatment (P = 0.105). In non-CIMT group, the mean post-operative hearing thresholds were significantly better at two weeks post-treatment (42.86 ± 24.87 dB) and four weeks post-treatment (30.34 ± 22.93 dB) than they were pre-operatively (62.48 ± 25.11 dB, both P <0.001). The mean hearing threshold at four weeks post-treatment (30.34 ± 22.93 dB) also presented a significant improvement over the hearing threshold at two weeks post-treatment (42.86 ± 24.87 dB, P <0.001).

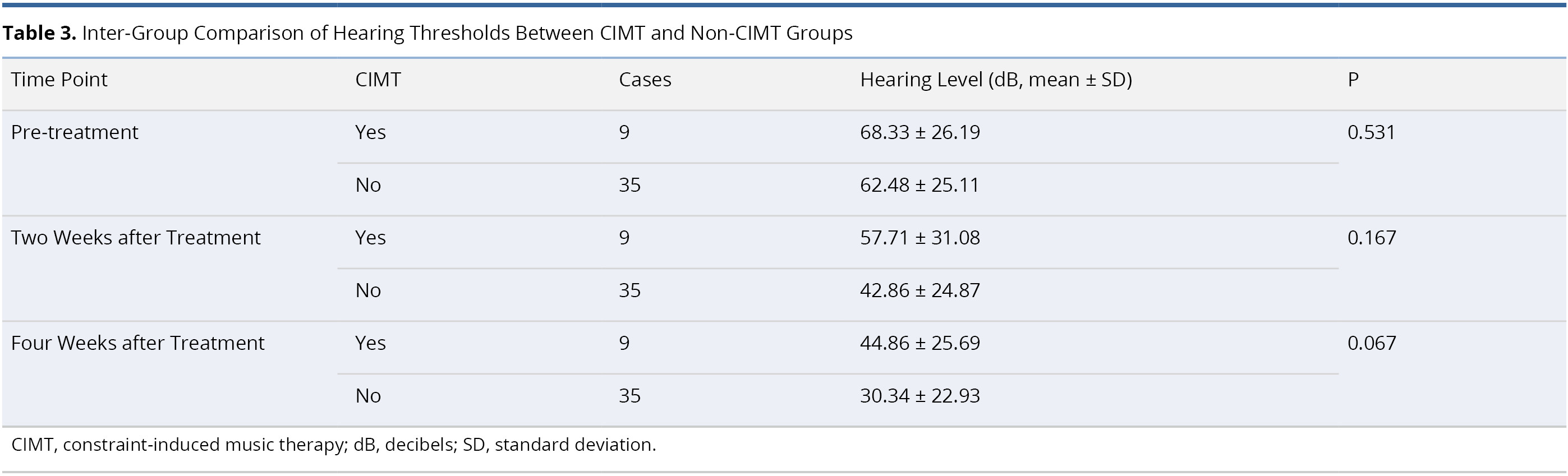
Table 3 presents an inter-group comparison of audiometric results between the CIMT and non-CIMT groups. The two groups were comparable in terms of mean pre-treatment hearing threshold (68.33 ± 26.19 dB in CIMT group and 62.48 ± 25.11 dB in non-CIMT group, P = 0.531). No statistically significant differences were observed between the two groups with regard to the mean hearing thresholds at two weeks post-treatment (57.71 ± 31.08 dB in CIMT group and 42.86 ± 24.87 dB in non-CIMT group, P = 0.167) and four weeks post-treatment (44.86 ± 25.69 dB in CIMT group and 30.34 ± 22.93 dB in non-CIMT group, P = 0.067).

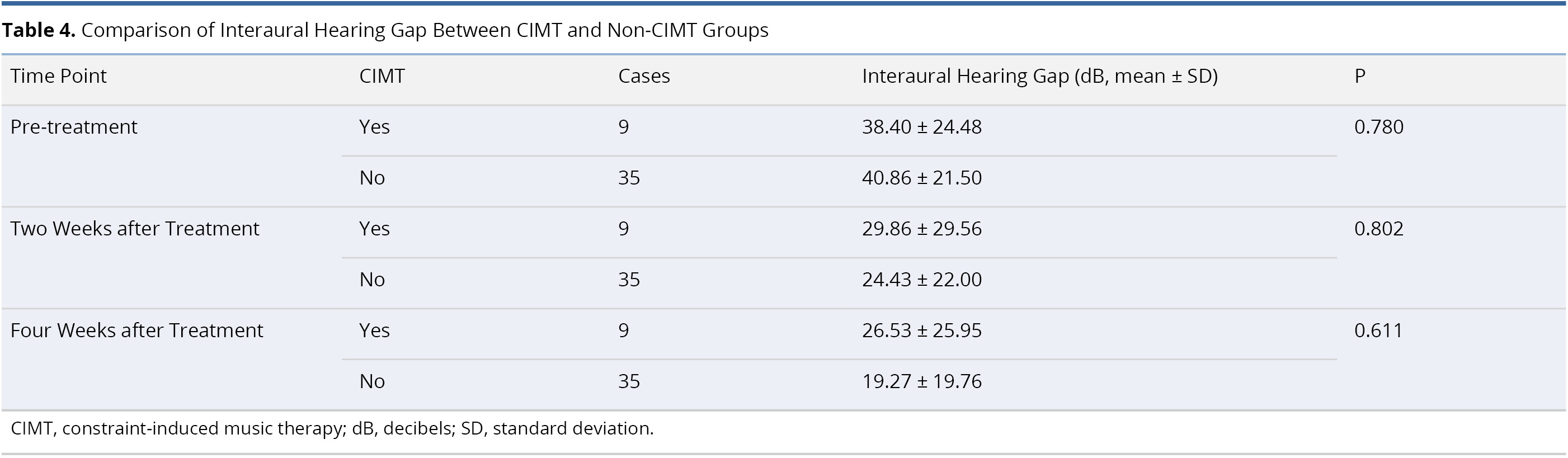
Table 4 lists an inter-group comparison of differences in interaural hearing threshold between the CIMT and non-CIMT Groups. The two groups were comparable prior to treatment (interaural hearing gap 38.40 ± 24.48 dB in CIMT group and 40.86 ± 21.50 dB in non-CIMT group, P = 0.780). After treatment, we were unable to detect statistically significant differences in interaural hearing gap between the two groups at two weeks post-treatment (29.86 ± 29.56 dB in CIMT group and 24.43 ± 22.00 dB in non-CIMT group, P = 0.802) or four weeks post-treatment (26.53 ± 25.95 dB in CIMT group and 19.27 ± 19.76 dB in non-CIMT group, P = 0.611).

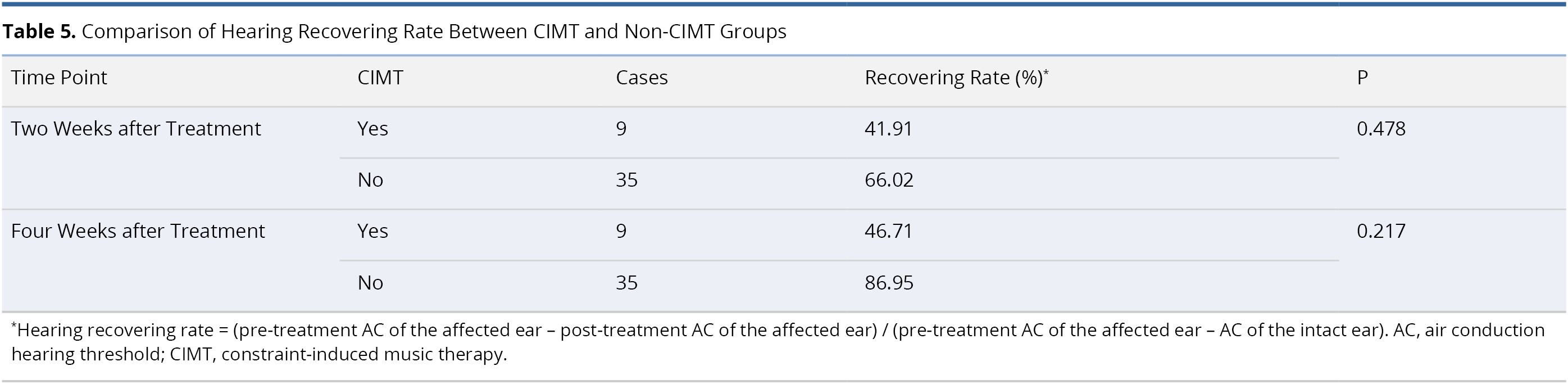
Table 5 presents a comparison of hearing recovering rates between the CIMT and non-CIMT groups. No statistically significant differences were observed in the hearing recovering rate between the two groups at two weeks post-treatment (41.91% in CIMT group and 66.02% in non-CIMT group, P = 0.478) or four weeks post-treatment (46.71% in CIMT group and 88.95% in non-CIMT group, P = 0.217).

Table 6 lists the results of multivariate logistic regression used to identify potential predictors of hearing recovery at two and four weeks post-treatment. This analysis revealed no statistically significant correlations between post-treatment hearing recovery and the application of CIMT (odds ratio, OR, 3.84, confidence interval, CI, 0.18 - 81.65, P = 0.388 at two weeks post-treatment; and OR = 2.70, CI = 0.15 - 47.60, P = 0.497 at four weeks post-treatment). It was observed that patients with better pre-treatment hearing level (≤60 dB) were more likely than those with worse hearing (>60 dB) to present hearing recovery at two weeks (OR = 125, CI = 4.88 - 3181, P = 0.004) and four weeks (OR = 45.41, CI = 3.60 - 573, P = 0.003) post-treatment. The other three factors (age, gender, and side of ear) did not have a significant influence on hearing recovery at two or four weeks post-treatment.

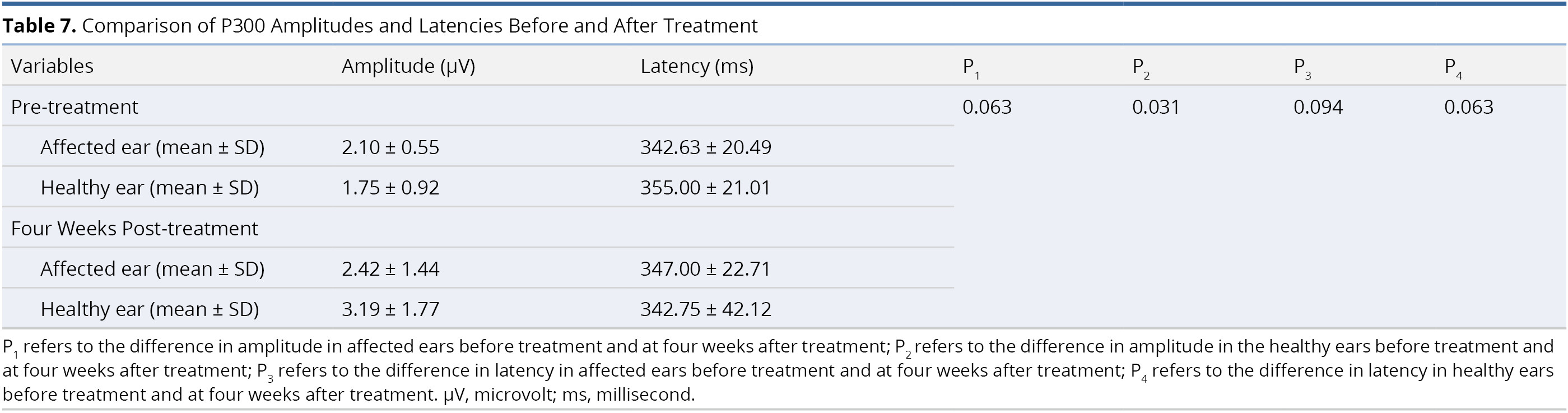
Table 7 presents a comparison of the amplitude and latency values of the P300 component in each ear before and after treatment. We succeeded in recording the P300 component in 66.7% of the subjects (6 of 9 patients). There was a significant difference in amplitude value in the healthy ears before treatment (1.75 ± 0.92 μV) and at four weeks after treatment (3.19 ± 1.77 μV, P = 0.031). We observed no other statistically significant differences in P300 amplitudes or latencies in the affected or healthy ears (all P > 0.05, Table 7).

Main Findings
In both the CIMT and non-CIMT groups, we observed a significant improvement in hearing thresholds at two weeks and four weeks post-treatment (Table 2). Nevertheless, we failed to detect any statistically significant differences between CIMT and non-CIMT groups in terms of hearing recovery when comparing outcome variables, including hearing threshold (Table 3), interaural hearing gap (Table 4), and hearing recovering rate (Table 5). Patients who underwent CIMT in conjunction with conventional steroid therapy were no more likely than patients who received only steroid therapy to recover hearing to normal limits (Table 6). In a subgroup assessment of 6 patients, the long-latency auditory evoked potential did not provide any significant indications of neuroplasticity in response to CIMT (Table 7).
New Therapeutic Direction for SSNHL: Neuroplasticity
One set of clinical practice guidelines for SSNHL in the US advises against the routine prescription of antivirals, thrombolytics, vasodilators, vasoactive substances, or antioxidants for patients with SSNHL. Furthermore, the benefit/harm balance for steroid therapy remains uncertain [1]. Considering the devastating effects that SSNHL can have on the patient’s quality of life, devising a new therapeutic direction to deal with SSNHL is no doubt a worthy endeavor [18].
Neuroplasticity can be viewed as adaptive responses to conditions associated with a behavioral gain, such as learning. Advances in our understanding of brain plasticity have led to the development of promising interventions for several neurological conditions and disorders. These methods are meant to promote adaptive neuroplastic changes to compensate for lost functions or to maximize remaining functions [43-45]. Results from numerous human and animal studies indicate that there are strong links between the expenditure of effort in training and use-dependent structural adaptation in the brain [46-50]. Plastic changes in brain structure contribute to an adaptive gain in function in healthy individuals (e.g., learning skills or creating memories) as well as recovery from brain damage. Cortical reorganization has been shown to compensate for loss of function or increase residual functions following brain lesion. Neuroplasticity can relieve initial deficits in behavior as well as perceptual and cognitive skills [51]. Plasticity-promoting theory has been implicated to achieve clinical gains and improve behavioral outcomes in parallel with increased brain plasticity. Several interventions have been developed to enhance brain plasticity, such as Hebbian learning [52], task-specific training [53], transcranial magnetic stimulation [54], deep brain stimulation [55], cognitive behavioral therapy [56], physical training [57], and neuropharmacotherapies that involve the molecular manipulation of numerous cellular and synaptic pathways [19,58,59].
Adaptive plasticity refers to a neural change in a positive direction for behavioral gain (e.g., skill learning) or functional compensation (e.g., post-stroke recovery). However, other forms of plasticity can induce maladaptive cortical reorganization, such as focal hand dystonia [30], phantom limb pain [60], and tinnitus [61,62]. These can have a negative effect on disease pathogenesis, resulting in unanticipated and undesired consequences [19,22,51,63-65]. Dealing with these adverse consequences requires interventions aimed at blocking or hindering plasticity, such as behavioral training to overcome focal hand dystonia [30], phantom limb pain [60], and tinnitus [61].
Additive Effect of CIMT on Hearing Recovery
It is possible that gains in functional recovery could be enhanced by preventing maladaptive plastic changes [18], and a promising therapeutic start may indicate a new direction for the management of SSNHL.
Based on previous findings, it is reasonable to expect spontaneous recovery in a considerable proportion of patients (32% to 65%) [2,5,14]. However, many patients face permanent hearing deficit, which brings on frustration, anxiety, insecurity, loneliness, depression, and social isolation [1,66]. This treatment limitation and potentially serious consequences highlight the need to aggressively explore a new avenue for therapeutic approach.
In animal experiments, researchers observed that a decrease in the spontaneous and driven firing rate in auditory nerve fibers following acoustic trauma commonly triggers central reorganization [26,27,67]. Researchers have also discovered that animals that undergo post-traumatic acoustic stimulation are less affected by hearing loss associated with hair cell damage to the cochlea, compared to animals that do not undergo such stimulation [23,24,26]. In other words, acoustic energy delivered to ciliated cells in the cochlea can be converted into electrical impulses that are transmitted to the auditory cortex through auditory nerves [68]. It has been surmised that acoustic stimulation (1) compensates for a loss of afferent neural inputs and (2) prevents maladaptive neuroplasticity in the brain. Both of these effects could promote hearing recovery [24,25].
In human studies using functional magnetic resonance imaging (fMRI) [69,70], many patients with SSNHL present an altered auditory cortical response under auditory stimulation. Magnetoencephalographic (MEG) studies have also revealed immediate and protracted changes in the function of auditory pathways among patients with SSNHL [71-75]. Overall, human studies support the findings of animal studies pertaining to SSNHL-induced brain plasticity.
In 2012, López-González et al. compared the outcomes of 65 SSNHL patients treated with medications (steroids, piracetam, and antioxidants) and 67 patients treated with medication as well as sound therapy (a combination of music and speech). They reported much higher hearing recovery among patients that underwent stimulation via sound therapy, compared to those without acoustic stimulation [68]. However, that study was based solely on audiometric outcomes. The absence of neuroimaging results (e.g., fMRI and MEG) precludes an examination of the extent and degree of plastic changes in the brain in response to SSNHL.
In 2014, Okamoto et al. [22] adopted a neuro-rehabilitation approach well-established in treating stroke patients (constraint therapy or constraint-induced movement therapy) in an attempt to enhance hearing recovery in patients with SSNHL [76]. All 53 of the SSNHL patients received traditional corticosteroid therapy, 22 of whom were also administered CIMT. They reported that the interaural hearing gap in the CIMT group was significantly smaller than that seen in the non-CIMT group between 1 and 6 months after treatment. In the current study, the interaural hearing gap in the CIMT and non-CIMT groups was smaller at two and four weeks after treatment. However, we did not observe statistically significant differences in interaural hearing gaps between the two groups (Table 4).
In both groups, we observed a significant improvement in hearing thresholds at two weeks and four weeks post-treatment (Table 2); however, there were no statistically significant differences in post-treatment hearing thresholds between the two groups (Table 3). This outcome was confirmed by multivariate analysis, which failed to detect a predictive association between the application of CIMT and hearing recovery to normal limits (Table 6). Okamoto et al. [22] did not compare post-treatment hearing thresholds between CIMT and non-CIMT groups.
We also compared outcomes in the two groups in terms of hearing recovering rate (Table 5). No significant intra-group differences were observed in hearing recovering rate. In 2015, Liu et al. [41] obtained similar results between an experimental group (47 patients receiving methylprednisolone and relaxing music) and a control group (42 patients receiving only methylprednisolone). Nonetheless, Liu et al. reported that acoustic treatment combined with steroid therapy alleviated the symptoms of SSNHL-related anxiety. In the current study, all 9 of the patients in the CIMT group reported a reduction in the stress and anxiety associated with SSNHL to tolerable levels.
Our findings do not provide audiometric evidence of an additive effect of CIMT on hearing recovery; however, we were able to demonstrate a beneficiary effect on the relief of stress and anxiety.
Degree of Neuroplasticity
Okamoto et al. [22] selectively included 6 patients in a CIMT subgroup for the analysis of neural activity using MEG, including an N1m response (generated mainly in the belt and parabelt areas of auditory cortex) [77] and auditory steady state response (ASSR, generated in the primary auditory cortex) [78]. Their results demonstrated a contralateral dominance of neural activity in both the primary auditory cortex (ASSR) and auditory belt areas (N1m) following steroid treatment in conjunction with CIMT. Dominance in the contralateral hemisphere is generally also found in healthy subjects with normal hearing [7,75]. Okamoto et al. claimed that the contralateral dominance of neural activity was an indication that SSNHL-induced contralateral cortical maladaptive plastic changes had been reversed. However, it may be premature to assert an association between hearing recovery and the reversal of cortical reorganization. Furthermore, audiometric outcome data of the 6 patients were not included in their report.
In the current study, we investigated post-treatment neuroplasticity by measuring the P300 component of long-latency auditory evoked potential. P300 is seen as a promising assessment tool for the detection of central changes in patients with sensorineural hearing loss [79-81]. We succeeded in recording the P300 component in 6 of the 9 patients studied (66.7%), which is consistent with the rates reported in the literature [80]. We observed a statistically significant difference in the amplitude values obtained from healthy ears before treatment and four weeks after treatment (P = 0.031, Table 7). However, when comparing the amplitudes and latencies of the affected ears (before treatment and four weeks after treatment), no significant changes were found (both P >0.05). Our electrophysiological findings are not in agreement with those reported by Okamoto et al. [22]. Nevertheless, we must not jump to any conclusions regarding the neuroplastic effects of CIMT on hearing recovery in patients with SSNHL. The sample sizes in the current study (6 patients) and in the study by Okamoto et al. (6 patients) [22] were of insufficient size to avoid bias in our sampling. A large number of patients will be required to determine the true degree of neuroplasticity in patients receiving CIMT.
Study Limitations
To the best of our knowledge, this was only the second study aimed at validating assertions that CIMT can prevent or reverse SSNHL-induced maladaptive cortical reorganization, thereby potentiating subsequent hearing recovery. Nonetheless, the current study was subject to a number of limitations. First, a larger sample size will be required to avoid sampling bias. Second, we acknowledge that bias may have been introduced at any stage of the study, due to the inclusion of a retrospective study arm. Selection bias (e.g., a non-representative study population) and information bias (e.g., imprecise measurements or incorrect recording of outcomes) could be avoided by adopting a double-blind, randomized, placebo-controlled trial in the future. All patients in the study received current first-line treatment (i.e., steroid therapy); therefore, it is unlikely that the functional recovery can be attributed solely to CIMT. Steroid therapy of this type has been shown to have success rates of 50 to 80% [11]. Outcome analysis was further complicated by spontaneous recovery, which has been reported in 32% to 65% of all cases of SSNHL [2,5,14]. Thus, it would be very difficult to determine the degree to which CIMT treatment exceeds spontaneous recovery. According to the literature, spontaneous improvement in hearing occurs primarily within two weeks after onset, and late recovery is highly unlikely for patients who do not undergo treatment. In the future, researchers should recruit volunteer patients who maintain the same level of hearing loss and who do not receive treatment for a period of two weeks following diagnosis with idiopathic SSNHL. A delayed treatment group of this sort could help to clarify the true effectiveness of CIMT on hearing recovery by eliminating the confounding effects of spontaneous recovery.
This study confirms that CIMT is a safe and cost-effective addition to corticosteroid treatment for SSNHL; however, our preliminary results could neither identify the additive effects of CIMT on hearing recovery nor confirm the degree of neuroplasticity in patients with SSNHL. Nevertheless, it appears that CIMT provides an enjoyable adjunct to conventional steroid treatment for the relief of stress and anxiety associated with SSNHL.
Received date: November 17, 2018
Accepted date: November 12, 2021
Published date: April 10, 2019
Dr. Kuo thanks Mr. Yi Chen for his assistance in completion of audiometric and electrophysiological examinations.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was sponsored by grants from the Medical Affairs Bureau Ministry of National Defense (MAB-107099) and Taoyuan Armed Forces General Hospital (AFTYGH-10734).
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2019 The Author. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).

This article unveils the hidden treasures within medical case reports, showcasing their historical significance, real-world relevance, and capacity to inspire innovation. By dissecting eight pivotal factors influencing the fate of case reports in contemporary academic publishing, it offers readers a profound understanding of the challenges and opportunities these narratives present. Readers will gain an enhanced appreciation for the essential role of case reports in linking clinical practice with research, highlighting rare conditions, new treatments, and unusual clinical cases. Furthermore, this article provides practical strategies for journals and the academic community to promote inclusivity, balance, and excellence in the publication of case reports, making it a must-read for anyone in the medical field.
This current review addresses the topic of therapeutic strategies for idiopathic SSNHL from the perspective of neuroplasticity. Assertions pertaining to the plausibility of this approach are based on a large body of evidence from animal experiments and recent studies on humans.
Newly discovered epidemiological evidence linking cholesteatoma to depression suggests that routine screening and monitoring of psychological status among cholesteatoma patients is warranted. Policies aimed at the early detection and timely treatment of comorbid depression following diagnosis with cholesteatoma could enhance health promotion and disease prevention.
The clinical significance of otitis media with effusion (OME), a complication associated with cleft lip/palate (CLP), is often overlooked in children. The author reviews the pathogenesis, clinical manifestations, and diagnoses of OME in children with CLP as well as the controversies surrounding treatment. He also provides a flowchart to guide the management of OME in children with CLP.
According to this article, Meniere's disease might actually be a generic term referring to an inner ear disorder that causes vertigo and hearing loss. The author suggests that physicians should pay more attention to discussing any pathological changes associated with hearing loss and vertigo with patients rather than narrowly focusing on confirming the diagnosis of diseases.
This editorial analyzes the extensive social and familial effects of rigorous educational demands in Taiwan, illustrated by a case study of a 45-year-old female music teacher with sudden sensorineural hearing loss. Forced to skip her medical appointments due to her child's demanding academic schedule, the incident highlights the profound impact of intense educational routines on family life. It critiques the prioritization of academic achievement over well-being, which places undue stress on both children and parents, advocating for a reevaluation of the emphasis on scholastic success versus holistic development. The piece calls for educational reforms towards nurturing, developmentally appropriate strategies that support natural growth and foster a lifelong love for learning. It promotes innovative teaching approaches, early intervention, and strategic use of educational technology to enhance learning outcomes, urging systemic changes to better prepare students for today's complex world. Overall, this is a commendable editorial that offers valuable insights into the need for educational reform.
Most isolated syndromes of Eight-and-a-Half Syndrome are associated with vascular etiology. Symptomatic trigeminal neuralgia due to infarction is rare. The author reports a patient with left-sided facial pain. It was followed by one-and-a-half syndrome with facial nerve palsy during the next day. Diffusion-weighted magnetic resonance imaging of his head revealed restricted diffusion in the left inferior pontine tegmentum neighboring the fourth ventricle extending ventrally. This case is the first report of Eight-and-a-Half Syndrome presented with recurrent attacks of unilateral facial pain, fulfilling criteria for classical trigeminal neuralgia.
This current review addresses the topic of therapeutic strategies for idiopathic SSNHL from the perspective of neuroplasticity. Assertions pertaining to the plausibility of this approach are based on a large body of evidence from animal experiments and recent studies on humans.
This editorial analyzes the extensive social and familial effects of rigorous educational demands in Taiwan, illustrated by a case study of a 45-year-old female music teacher with sudden sensorineural hearing loss. Forced to skip her medical appointments due to her child's demanding academic schedule, the incident highlights the profound impact of intense educational routines on family life. It critiques the prioritization of academic achievement over well-being, which places undue stress on both children and parents, advocating for a reevaluation of the emphasis on scholastic success versus holistic development. The piece calls for educational reforms towards nurturing, developmentally appropriate strategies that support natural growth and foster a lifelong love for learning. It promotes innovative teaching approaches, early intervention, and strategic use of educational technology to enhance learning outcomes, urging systemic changes to better prepare students for today's complex world. Overall, this is a commendable editorial that offers valuable insights into the need for educational reform.
Authors have put forth a hypothesis that the brain bears the innate capability of performing high-level mathematical computing in order to perform certain cognitive tasks. Authors give examples of Orthogonalization and Fourier transformation and argue that the former may correspond to the physiological action the brain performs to compare incoming information and put them in categories, while the latter could be responsible for the holographic nature of the long-term memory, which is known to withstand trauma. Authors plead that this proposal may not be as strange as it may appear, and argue how this line of mathematical modeling can have far-reaching consequences.
This current review addresses the topic of therapeutic strategies for idiopathic SSNHL from the perspective of neuroplasticity. Assertions pertaining to the plausibility of this approach are based on a large body of evidence from animal experiments and recent studies on humans.
Kuo CL. Neuroplastic effect of constraint-induced music therapy on hearing recovery in patients with sudden sensorineural hearing loss. Neurol Neurosci Res 2019;2(1):3. https://doi.org/10.24983/scitemed.nnr.2019.00110