Sinonasal teratocarcinosarcoma is an extremely rare malignant growth that arises in the nasal cavity. This tumour is the product of a combination of epithelial and mesenchymal tissues. A polypoidal mass was found in the right nostril of a 40-year-old male patient who had presented with complaints of right-sided nasal obstruction and recurrent epistaxis for the past six months. Biopsy results confirmed the diagnosis and resection of the mass was done using a lateral rhinotomy approach. Post-surgical histopathological testing further validated the diagnosis. Postoperative radiotherapy and chemotherapy were recommended by the oncologist, along with follow-up scans after 6 weeks.
Video Abstract
Sinonasal teratocarcinosarcoma (SNTCS) is an unusual and infrequently recognized malignant tumour believed to be found almost exclusively in the sinonasal area. It was first described by Shanmugaratnam in 1983 as teratoid carcinosarcoma [1] constituting several components of neuroectodermal, epithelial, and mesenchymal origins at varying stages of differentiation. These lesions are known to be extremely aggressive and commonly recurrent, with an average survival period of less than two years. Generally, due to the poor prognosis, most cases do not exhibit beyond a five-year survival period [2,3]. Histologically, this tumour seems to arise most probably from a primitive cell in the olfactory membrane that not only replicates the neuroectodermal features of olfactory neuroblastoma but also holds the potential to mature into many different types of somatic cells. Benign and malignant epithelial, mesenchymal, and neural elements are typically present in this tumour. Within this growth, the presence of non-differentiated tissue can be a likely finding. These immature tissues can also exhibit features similar to blastoma cells [2].
A forty-year-old male presented to the Outpatient Department with right-sided nasal blockage and recurrent epistaxis for the last six months. The patient was also suffering from episodic headaches, anosmia, and visual defects in the right eye.
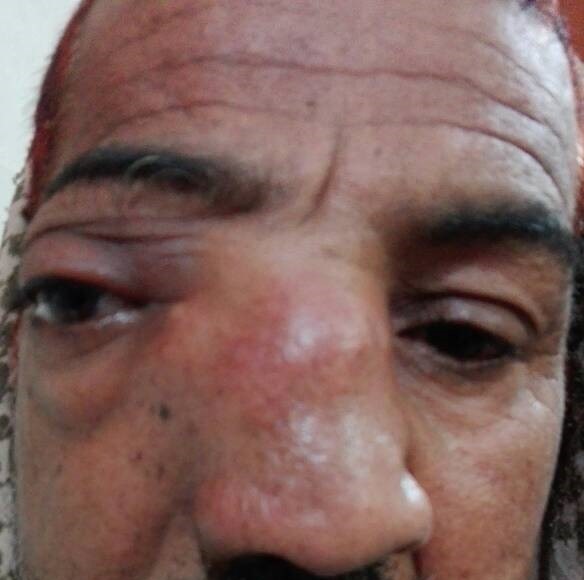
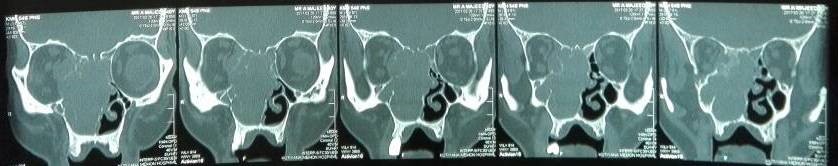
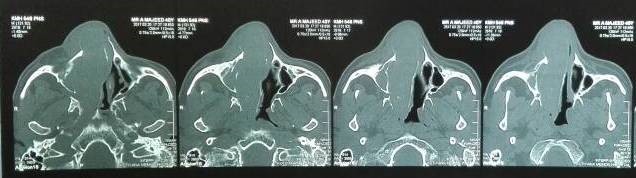
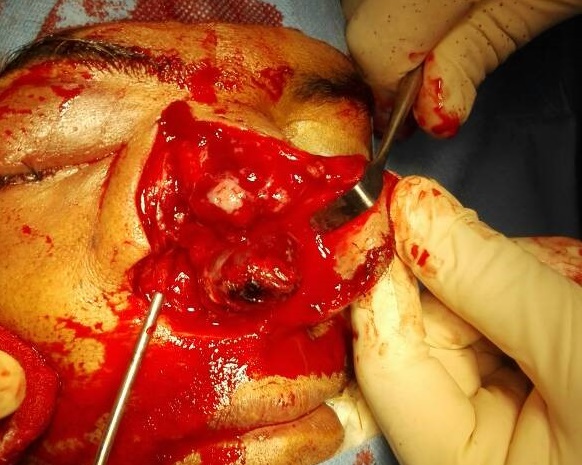
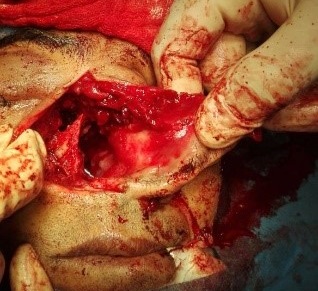
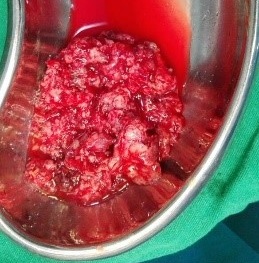
Under examination as shown in Figure 1, a large swelling on the right nostril was noted, which appeared to be extending towards the right eye. There was visible proptosis of the right eye and subsequent anterior rhinoscopy allowed visualization of a polypoidal mass and numerous blood clots within the right nostril. CT scan of the sinuses (Figure 2 and 3) revealed a mass in the right nostril extending from the roof of the nose to the right eye and involving most of the cribriform plate. The mass was noted to be breaching the lamina papyracea (or orbital lamina), a smooth and oblong bone plate, which forms the lateral surface of the ethmoid bone in the skull. Furthermore, the cause of proptosis was discovered to be the involvement of the medial rectus muscle of the eye being pushed upwards almost to the optic nerve. Lymph nodes of the neck were not palpable on examination or visible on chest x-ray. The grading of the tumour was therefore established as T3 N0 M0 . The tumour was resected through a lateral rhinotomy approach. Resection of the entire tumour (Figure 4), measuring 8.5 x 7 cm (Figure 5), was successful and the mass was sent for histopathological testing (Figure 6). Analysis of the mass showed an extensively necrotic tissue consisting of numerous polymorphonuclear cells and lymphocytes. Interspersed within lymphocytes are sheets of spindly cells with occasional prominent nucleoli and several apoptotic cells. Lymphovascular invasion was not identified. Cytokeratin AE1/AE3 was diffusely positive, Cytokeratin 7 was negative, and leukocyte common antigen (LCA) was negative. The diagnosis was proven to be of Sinonasal teratocarcinosarcoma.
After post-surgical care, the patient was referred to the Oncology department where radiotherapy of 6000 cGy was recommended. Due to the decreased sensitivity of this tumour to chemotherapy, it was not initiated for this patient.
The patient’s presenting symptoms of nasal blockage, epistaxis and headaches appeared to have greatly improved post-operatively. There has also been a slight improvement in the right-sided vision and his sense of smell. The patient will be monitored closely on follow-up due to the high rate of recurrence.
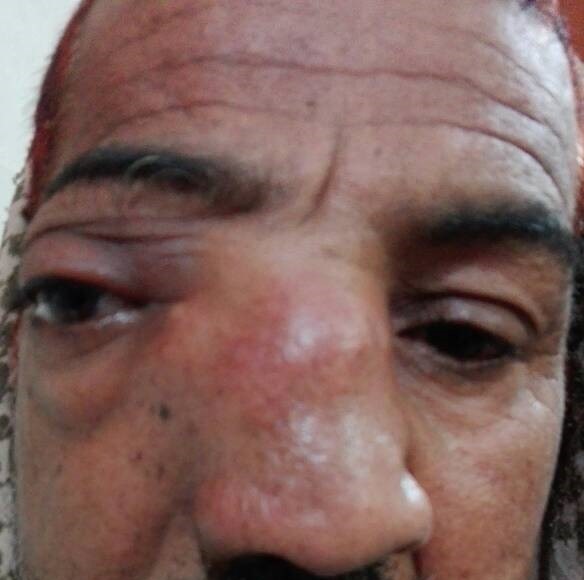
Figure 1. Initial presentation of patient (nasal swelling, deviation of right eye).
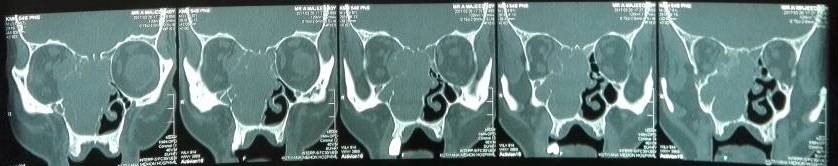
Figure 2. Coronal section of CT scan sinuses and nasal cavity.
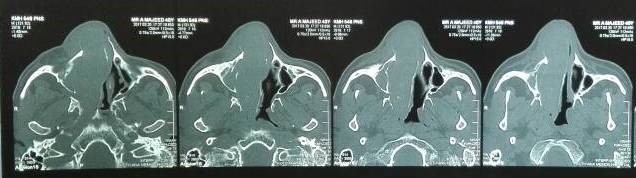
Figure 3. Axial sections of CT scan sinuses and nasal cavity.
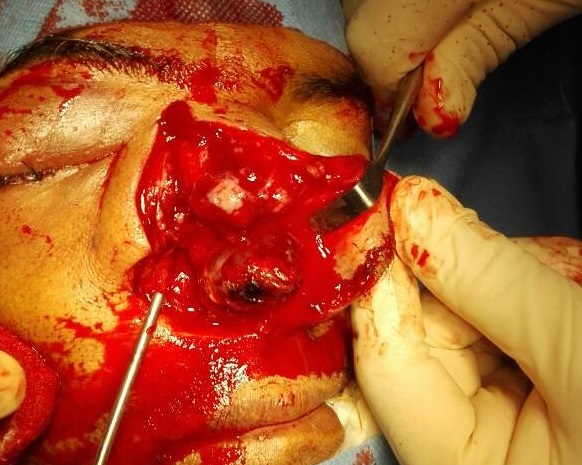
Figure 4. Intra-operative visualization of nasal tumour.
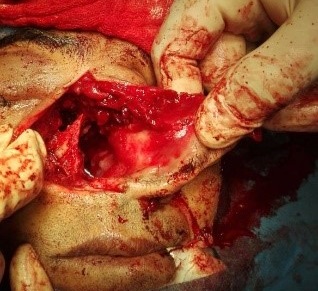
Figure 5. Intra-operative visualization of nasal cavity after removal of tumour.
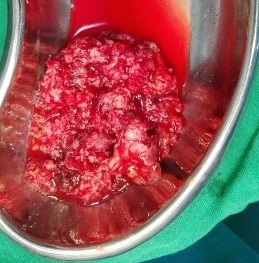
Figure 6. Tumour after removal from the patient.
The Sinonasal teratocarcinosarcoma is found more commonly in males rather than females, with the approximate ratio of 4:1. This lesion, almost as a rule, arises primarily in the ethmoid sinus and maxillary antrum [2]. The diagnosis of this growth, however, can only be truly confirmed by thorough sampling following resection. A preliminary clinical assumption of the diagnosis may be the sole way to navigate towards a differential diagnosis of SNTCS as opposed to poorly differentiated carcinomas, including sinonasal undifferentiated carcinoma, adenocarcinoma, neuroendocrine carcinoma, rhabdomyosarcoma, synovial sarcoma, olfactory neuroblastoma, and malignant mixed salivary gland tumours [4]. Malignant teratomas can generally be ruled out, unlike the benign teratoma, since they have an extremely low chance of arising in the sinonasal tract. Conversely, the lack of comprehensive testing may result in an eventual misdiagnosis such as olfactory neuroblastoma, squamous cell carcinoma, undifferentiated carcinoma, adenocarcinoma, malignant salivary gland-type tumours, or adenosquamous carcinoma [5].
The origin of the sinonasal teratocarcinosarcoma remains unknown for the most part. Although differentiation from a multipotential adult somatic stem cell, with differing levels of differentiation, has been hypothesized [3], this postulation has majorly been attributed to the histological absence of germ cell elements such as those of yolk sac, germinoma, embryonal carcinoma, or choriocarcinoma, along with a virtual non-appearance of any notable amplification of allele 12p, found mostly as isochromosome 12p (i12p). This is a finding most often noted in lesions with a germ cell origin [6].
The gold standard diagnostic method for this tumour is immunohistochemical staining. Epithelial components are cytokeratin and epithelial membrane antigen positive. Neuroepithelial components are neuron-specific enolase, CD99, chromogranin, synaptophysin, glial fibrillary acidic protein, and S-100 protein positive. Mesenchymal components are vimentin positive and can test positive for myogenic markers or actin, depending on the type of cell present [7]. As seen in this case, Cytokeratin AE1/AE3 was diffusely positive, Cytokeratin 7 was negative, and LCA was negative, showing that this type of tumour consisted primarily of epithelial components.
A fundamental case series on this lesion was described by Budrukkar et al. They studied the medical records of twenty-two patients (only one female) with histopathologically identified sinonasal teratocarcinosarcoma, which had been diagnosed from 1993–2007. Sixteen of these patients received complete treatment. Fourteen of them received surgery, followed up by radiation therapy and eleven cases were also given adjuvant chemotherapy. Only two individuals received chemoradiation as the definitive treatment, replacing surgery. The follow-up done on average at about thirty-four months in the remaining patients showed that only five cases had their disease under control. Recurrence was noted in eleven patients, with a median time of 7 months. The 2-year disease-free survival rate and the overall survival rate were 28% and 46%, respectively. This study concluded that a multifaceted approach in treatment is the most effective method of management. The best treatment involves a combination of surgery, radiation therapy and chemotherapy [8].
The most commonly seen form of treatment failure is the recurrence. The elevated incidence of local recurrence and metastasis is a very significant indicator of the aggressive nature of this tumour [9]. To combat this rapid growth rate, an elective neck dissection should be advised immediately in the earlier stages of the disease. Greater attention is necessary for any soft tissue in close proximity to the existing lymphadenopathy. This approach may lower cervical lymph node or distant metastasis [10]. Distant metastasis of SNTCS, however, is a rarely reported phenomenon due to the primarily invasive nature of the cancer. If present, metastasis to the lungs and airways can manifest as obstruction to the airway and present as dyspnoea [11].
One of the greatest challenges in this disease has been to obtain substantial evidence of an improved cure rate following chemotherapy owing to the rarity of incidence. As far as radiation therapy is concerned, however, prior studies were able to establish that the patients who did not receive postoperative radiotherapy faced a greater threat of recurrence as compared to those who received radiotherapy [12].
Sinonasal teratocarcinosarcoma is an extremely rare tumour that most commonly arises in males in the ethmoid sinus or maxillary antrum. In order to combat the high rate of recurrence, analysis of the resected mass and a further study of chemo and radiotherapeutic regimens according to histopathological findings are necessary for a fixed and effective treatment protocol to be established.
Received date: December 12, 2017
Accepted date: February 19, 2018
Published date: May 07, 2018
None
None
Written patient informed consent was obtained.
© 2018 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Video Video Abstract
Farooq W, Baig S. An unusual nasal mass: The sinonasal teratocarcinosarcoma. Arch Otorhinolaryngol Head Neck Surg 2018;2(1):1. https://doi.org/10.24983/scitemed.aohns.2018.00061