Introduction: Cyclooxygenases (COX) catalyze the synthesis of prostanoids from arachidonic acid, present as two isoforms, namely COX-1 and COX2. COX-2 has been shown to be associated with tumor progression in various cancer patients. The most prevalent type of cancer in the head and neck region is squamous cell carcinoma, with an overall poor outcome in part due to the early spread of metastatic cells.
Materials and Methods: Primary tumor specimens as well as lymph node specimens harvested during neck dissection of 289 patients with a diagnosis of HNSCC were analyzed and subjected to immunohistochemical and H-score analysis of COX-2 expression. Demographics, diagnoses, histopathology and succeeding outcome were subsequently analyzed.
Results: The primary cancer was squamous cell carcinoma in all patients (oral cavity n: 16, oropharynx n: 28, hypopharynx n: 11 and larynx n: 10 [stage III n=18; stage IVA n=45; stage IVB n=2]). H-score for COX-2 expression in the primary lesion as well as metastatic lymph nodes was significantly higher in the advanced stages compared with the early stages, with no significant differences among tumor locations. High COX-2 expression in primary lesions as well as metastatic lymph nodes was associated with poorer overall survival rates at a mean follow-up of 83.4 months (6 - 204 months).
Conclusion: COX-2 expression in HNSCC varied from the anatomical site, correlated positively with tumor stage and was associated with poor overall survival rates. Therefore, COX-2 expression in primary lesions as well as lymph node metastases appears to identify HNSCC patients at higher risk in all tumor sites. Adjuvant therapeutic approaches targeting COX-2 might be a promising tool in this patient population.
Head and neck cancer represents the sixth most common cancer worldwide with an incidence of approximately 630,000/year [1]. More than 95% of these tumors are squamous cell carcinomas (SCC). Despite advances in the major therapeutic areas, such as surgery, radiotherapy and chemotherapy, the survival rates for patients suffering from this disease have not significantly improved within the past decades [2]. Therefore, identifying new molecular targets in head and neck squamous cell carcinomas (HNSCC) might contribute to improving cancer treatment and patients’ overall prognosis.
Cyclooxygenases (COX) catalyze the synthesis of prostanoids from arachidonic acid. There are two isoforms of COX, namely COX-1 and COX-2 [3]. COX-1 is constitutively expressed in various cells, whereas COX-2 is an inducible enzyme. The induction of the COX-2 gene is stimulated by various factors, such as cytokines, oncogenes and carcinogens. It is reported to be predominantly induced and activated in numerous pathological conditions, such as inflammation and cancer [4,5]. It has been revealed from various studies that COX-2 is overexpressed in numerous human tumors, including HNSCC [6-8]. Further, COX-2 seems to play an important role in carcinogenesis and tumor progression, as it has been shown to be upregulated in transformed cells, premalignant as well as malignant lesions [9].
In oral squamous cell carcinoma, it has been shown by Pandey et al., that COX-2 expression has been significantly upregulated in OSCC compared to normal mucosa and oral dysplasia [10]. Further, COX-2 has been shown to promote tumor progression by inducing various pathways in critical stages of malignant disease [11]. Its overexpression and activity stimulate cell division, angiogenesis and metastases [12,13]. Studies suggest that COX-2 contributes to tumor progression by modulating the immune system to reduce anti-tumor immune responses [14,15]. Also, it has been suggested to act on tumor cells by promoting their mitotic activity and subsequently aid the conversion of premalignancy to invasive tumors [16]. Further, COX-2 has been described to induce angiogenic factors, such as vascular endothelial growth factor (VEGF) and fibroblastic growth factor, and therefore promotes tumor angiogenesis, tumor cell migration, and the formation of local and distant metastases [17,18]. Overexpression of COX-2 in primary tumor tissues has been shown to correlate negatively with patients’ outcome and positively with tumor progression and recurrence rates in numerous tumors, including HNSCC [19,20].
However, its role in lymph node metastases in HNSCC has not been well established so far. The aim of the present study was to investigate COX-2 expression in primary tumors and lymph node metastases in HNSCC patients of various tumor sites and correlate these to patients’ overall survival.
Patient Population
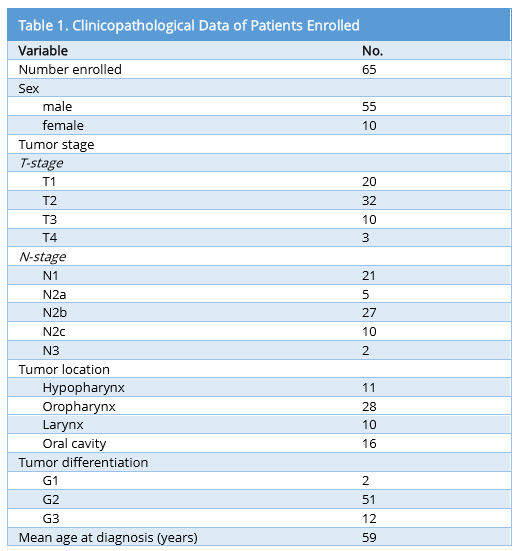
Following approval by the Institutional Review Board, the records of 289 patients, with a diagnosis of squamous cell carcinoma of the head and neck cancer between 1999 and 2012, were identified from an institutional database. Inclusion criteria were no prior treatment as well as surgical resection of the tumor, including at least a unilateral neck dissection for clinically metastatic disease. Patients, who did not undergo surgery, including a neck dissection as the primary treatment modality, were excluded from the study. Also, patients with a pathological N0 neck were excluded from the study. Altogether, 65 patients met these criteria and were included in the study.
Immunohistochemistry
Representative slides from each block were cut and stained as indicated below. Slides were reviewed by a pathologist (MR) to confirm histology. Blocks were sliced and deparaffinized sections were subjected to immunohistochemical staining using the following antibodies: anti-COX-2 (Novus Biological, Wiesbaden, Germany) and anti-mouse-IgG1 (DAKO, Santa Clara, CA, USA). Appropriate negative controls (without primary antibodies) were included for each specimen.
The staining was evaluated independently by two investigators blinded to the sample type. COX-2 expression was assessed in the primary tumor lesions as well as metastatic lymph nodes using the semiquantitative scoring method. Whole sections were observed under the microscope [low power (LP) and high power (HP)] and positive cells were counted in four successive fields selected randomly in primary tumor lesions as well as metastatic lymph node (400 × HP). The scoring standard for staining was described as follows: negative (0, no staining in an HP field), weakly positive (1, staining only in an HP field), moderately positive (2, staining in an LP field), and strongly positive (3, positive staining in an LP field). The scores of a section in four successive HP fields were averaged (0, positive cell percentage <5%; (1) positive cell percentage = 6–25%; (2) positive cell percentage = 26–50%; (3) positive cell percentage = 51– 75%; (4) positive cell percentage >75%). The scores of positive cell density and percentage were summed up to give the total score. For subgroup analysis, an H-score of ≤ 200 was considered “low” and an H-score of >200 was considered “high”.
Statistical Analysis
Statistical analysis was performed using the student t test to compare variables within groups. The Kaplan-Meier estimator was employed for survival analysis and the generated curves were compared with Cox’s Ftest. The endpoint for the study was overall survival (OS). OS was defined as the time from sample collection to death or censoring. Censoring was defined as loss of follow-up or alive at the end of follow-up. A p-value of ≤ 0.05 was considered statistically significant.
Patient Cohort
Patient demographics and tumor characteristics are shown in Table 1. 65 patients (55 males and 10 females; 59 ± 9 years) were retrospectively identified with a surgically treated tumor of the head and neck. The histology was squamous cell carcinoma in all patients, with 16 oral cavity lesions, 28 oropharyngeal, 11 hypopharyngeal and 10 laryngeal lesions.
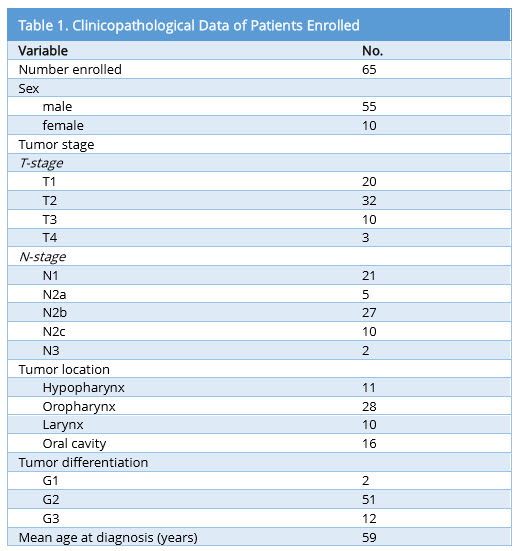
COX-2 expression in primary tumor lesions and lymph node metastases
COX-2 expression using the H-score was determined for primary tumor and lymph node metastases. Figure 1 shows representative staining for COX-2 in the primary lesion. Laryngeal lesions appear to show the highest extent of COX-2 expression in the primary lesions and lymph node metastases, however, without significant differences compared with other sites (Figure 2). Adjacent tissues, as well as normal mucosa, did not show any significant positivity for COX-2 (data not shown). As shown in Figure 3A, B and C, COX-2 expression correlated positively with T stage, N stage and tumor stage (Figure 3A, 3B and 3C).
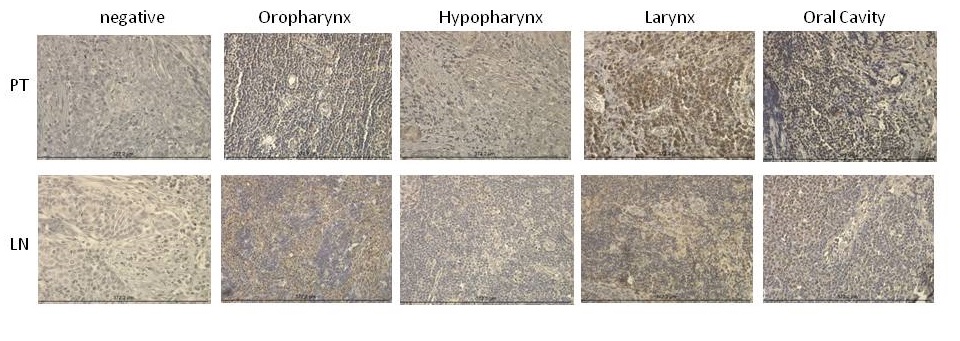
Figure 1. COX-2 staining patterns in primary tumor and lymph node specimens. Immunohistochemical analysis of COX-2 expression in primary tumor as well as lymph node specimens is shown. Representative slides of staining from the oropharynx, hypopharynx, larynx, and oral cavity with their lymph node specimens are shown respectively.
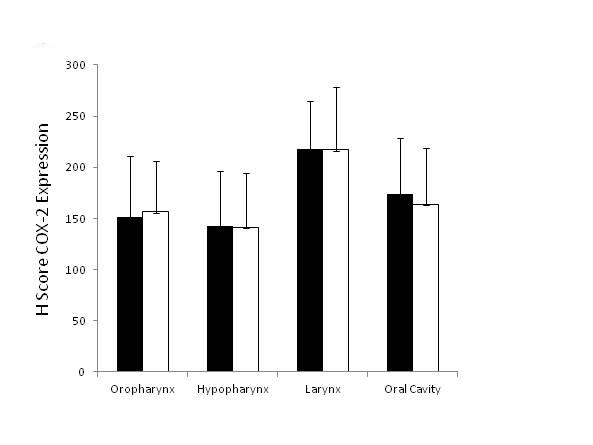
Figure 2. COX-2 expression in primary tumors and lymph node metastases in different HNSCC tumor locations. Primary tumor specimens and lymph node specimens harvested during a neck dissection were stained for COX-2 and immunohistochemically analyzed. Expression of COX-2 among different tumor locations was analyzed. The data are from 65 HNSCC patients.
Figure 3. COX-2 expression in primary tumors and lymph node metastases. (A) Primary tumor specimens were stained for COX-2 and immunohistochemically analyzed. The data are from 65 HNSCC patients. (B) Lymph node specimens harvested during a neck dissection were stained for COX-2 and immunohistochemically analyzed. The data are from 65 individuals with HNSCC tumors (C) Immunohistochemical analysis of COX-2 expression in primary tumor specimens as well as lymph node specimens according to tumor stages. The data are from 65 HNSCC patients. Asterisks indicate a p-value of < 0.05.
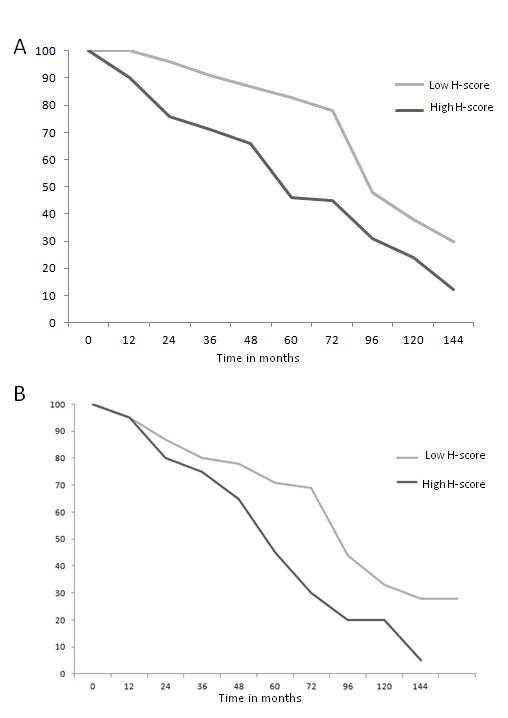
Figure 4. Survival analysis of HNSCC patients. (A) Kaplan-Meier overall survival curve, according to COX-2 expression (low versus high) in the primary tumor lesion. (B) Kaplan-Meier overall survival curve, according to COX-2 expression (low versus high) in the metastatic lymph nodes. The data are from 65 individuals with HNSCC tumors.
Correlation of infiltrates to prognosis and outcome
Next, we wished to analyze the correlation of COX-2 expression in primary tumors and lymph nodes with the overall survival of the patients. Therefore, patients with a low H-score for COX-2 expression were compared with patients with a high H-score, as described in Materials and Methods. As shown in Figure 4A, high COX-2 expression in primary tumor specimens was associated with shorter overall survival (OS) (p < 0.05). Also, high COX-2 expression in metastatic lymph nodes showed significantly decreased OS rates (p < 0.05) (Figure 4B). High H-scores for the primary tumor as well as lymph node specimens did not correlate to disease recurrence in the analyzed patient population (Figure 4A and 4B).
The results of the present study show a higher expression for COX-2 in the advanced HNSCC compared with the early-stage HNSCC in the primary tumor as well as metastatic lymph nodes. Further, our data suggests not only a negative correlation of COX-2 expression in primary tumor to patients’ overall prognosis in HNSCC, but also for COX-2 expression in metastatic lymph nodes to patients’ overall prognosis. An overexpression of COX-2 has been reported previously for many various tumor types, including HNSCC [6-8]. In some of these malignancies, overexpression of COX-2 is associated with poor prognosis and low survival rate [21,22].
In the present study, overexpression of COX-2 in primary tumors as well as metastatic lymph nodes was associated with decreased OS rates compared to low COX-2 expression. The mechanisms of COX-2 overexpression during carcinogenesis are still not well understood. However, it has been shown in other tumor types that COX-2 overexpression can be a result of the inactivation of the tumor suppressor genes, like p53 [23] or activation of certain proto-oncogene, such as Ras [24]. The formation of regional and distant metastases is favored by the capacity of tumors to progress locally, which subsequently invade lymphatic and blood vessels, and promote neo-angiogenesis. Morita et al. showed that COX-2 promotes tumor lymphangiogenesis and lymph node metastasis in oral squamous cell carcinoma [25]. In their study, they showed a positive correlation of COX-2 expression to VEGF-C expression, a potent stimulator of lymphangiogenesis. Prostaglandin E2, as a major product of COX-2 overexpression and activity, has also been shown to increase tumor cell proliferation, angiogenesis, invasion, and metastasis in various tumors [26]. Prostaglandin E2 has been shown to inhibit tumor necrosis factor α (TNFα) and induce interleukin-10 (IL-10), and therefore contributing to profound immunosuppression [27,28]. Based on these findings, targeting the COX2 pathway with selective COX-2 inhibitors seems to be a legitimate approach in anti-tumor therapy. Inhibitory effects of these agents on the growth and metastasis of tumors have been shown in various models [28,29]. Further, the positive effects of COX-2 inhibitors on the anti-cancer effects of radiation therapy as well as chemotherapy have been described in animal models [30,31]. Also, an increased expression of COX-2 in oral mucosal dysplasia and an overexpression in oral cancer reflect its role in the early stages of oral carcinogenesis as well as tumor progression [10]. In esophageal carcinoma, COX-2 overexpression was described to be associated with increased tumor invasion, more advanced tumor stages, poorer differentiation and prognoses, compared with cases with low COX2 expression levels [32,33]. These results all indicate a crucial role of COX2 in carcinogenesis and metastases.
In the present study, a positive correlation was found between the expression of COX-2 in advanced HNSCC of all sites, as well as a negative correlation to patients’ outcome. Higher expression levels of COX-2 were present in the patients with advanced primary tumors and advanced lymph node metastasis, compared with those patients with early tumors. The increased expression of COX-2 in the advanced primary, as well as the metastatic disease, may reflect its role in the development and progression of HNSCC. Thus, according to the obtained results, COX-2 may be used for the molecular target in adjuvant therapy in HNSCC. It seems that selective COX-2 inhibitors may be beneficial in preventing the transformation of premalignant lesions to malignancies, and further tumor progression as well as the formation of metastases. However, there appears to be a wide range of pathways and factors that were associated with tumor progression comparable to COX-2, such as e. g ectonucleotidases [34]. Therefore, further studies are required to identify patient populations, who would benefit from adjuvant treatment modalities targeting the COX-2 pathway.
COX-2 expression in primary lesions, as well as lymph node metastases, appears to identify HNSCC patients at higher risk in all tumor sites. Adjuvant therapeutic approaches targeting COX-2 might be a promising tool in this patient population.
Received date: June 08, 2017
Accepted date: July 10, 2017
Published date: August 10, 2017
© 2017 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
In the present manuscript, the authors compared the expression of COX-2 in the primary lesion from different head and neck regions and the relevant metastatic lymph nodes. They found that the differential expression of COX-2 was related to the clinical stage and differentiation of tumor, not the tumor location in HNC.
The earliest relevant article appeared in 2001. The authors showed that COX-2 pathway had a central role in HNC angiogenesis by modulating VEGF production and COX-2 inhibitors might be useful in HNC treatment. More articles have emerged in past 10 years. Oqawa et al. reported that the appropriately selective administration of certain COX-2 inhibitors had an anti-metastatic effect through suppression of the EMT by restoring E-cadherin expression in HNSCC. They added that the down regulation of CDH-1 resulting from the EMT might be closely involved in lymph node metastasis. So far, the clinical significance of COX-2 pathway and the related molecular mechanism in HNC have been widely reported. However, further research is necessary regarding the various mechanisms underlying the anti-cancer effects of COX-2 inhibitors against tumors.
In Figure Legends section, Figure 2 is the same as Figure 3 completely. The authors should revise it.
ResponseThe figure legends have been revised as suggested.
In Results-“COX-2 expression in primary tumor lesions and lymph node metastases”, the description of the section is confusing, especially the figure explanation. The presentation and explanation with their data need to be improved. The manuscript should be carefully revised.
Response The mentioned result section is carefully revised and the figures labelled accordingly.
The authors should provide the COX-2 staining image of lymph node in Figure 1.
Response The requested staining has been added to Figure 1.
The authors should provide the COX-2 staining images from oral cavity, oropharyngeal, hypopharyngeal, laryngeal, and the relevant lymph nodes, respectively.
Response Staining sections for each of the different tumor locations (oral cavity, oropharynx, hypopharynx, and larynx) have been added to figure 1 with representative sections for the primary tumor as well as lymph node metastases as requested.
I have gone through the revised manuscript. The authors have provided the necessary data and made proper modifications. I think the revised version is suitable for publication in AOHNS.
The new title has been much improved.
Boduc M, Roessler M, Mandic R, Netzer C, Güldner C, Walliczek-Dworschak U, Mandapathil M. Presence and quantity of COX-2 in lymph node metastases of patients with head and neck cancer. Arch Otorhinolaryngol Head Neck Surg 2017;1(2):1. doi:10.24983/scitemed.aohns.2017.00024