The objective of the study was to evaluate the use of systemic bevacizumab as an adjuvant treatment option for young patients presenting with recurrent respiratory papillomatosis (RRP). Two young children with aggressive RRP were retrospectively reviewed. Both patients underwent over ten surgical debridements and had recurrence of severe airway obstruction prior to receiving systemic bevacizumab infusions at 10 mg/kg intravenously. Post-infusion laryngoscopy with photo documentation was performed to evaluate treatment response. Patient 1 was diagnosed with RRP at 2 years of age and required debridements at 1- to 3-month intervals prior to starting bevacizumab infusions at monthly intervals for severe airway obstruction. Patient 2 was diagnosed with RRP at 5 months of age and required surgical debridements at intervals of 3-8 weeks with severe airway obstruction each time prior to receiving bevacizumab. After a single infusion, both patients were found to have no evidence of disease on follow up laryngoscopy. The patients remain disease free after 16 and 10 months post initial infusion respectively. In conclusion, systemic bevacizumab shows immense promise as an adjuvant therapy for young children with aggressive RRP necessitating frequent debridements for severe airway obstruction. However, further studies are required to determine ideal candidacy for this therapy as well as for determining optimal dosing and duration of treatment.
Recurrent respiratory papillomatosis (RRP) is a chronic disease characterized by exophytic proliferation of papillomas of the larynx and aero-digestive tract [1]. It is typically caused by Human papillomavirus (HPV) subtypes 6 and 11 and is thought to be transmitted through contact with secretions of an infected birth canal during childbirth [2]. It is the most common benign pediatric laryngeal neoplasm, affecting an estimated 1.7 to 2.3 per 100,000 children in the U.S, often presenting between the ages of 2 to 3 years old [3-5]. Although RRP is generally benign, papillomatous growths within the larynx frequently cause varying degrees of airway obstruction, necessitating multiple surgical debridements to restore airway patency.
RRP has historically been challenging to treat because of the recurrent nature of the disease. On average, children with RRP require 4.4 surgeries per year to maintain airway patency [6]. Furthermore, 3% to 7% of cases transform to squamous cell carcinoma, highlighting the importance of aggressive management [7]. Several adjuvant therapies, such as cidofovir and interferon, have been used in conjunction with surgery to treat more aggressive forms of papillomatosis; however, their ineffectiveness in eliminating disease as well as their potential adverse side effects make them incomplete treatment options [8].
Bevacizumab is an anti-angiogenesis drug that is currently being explored as an adjuvant therapeutic option for children with aggressive disease. Although originally described as an intralesional treatment modality, systemic delivery of bevacizumab has led to favorable outcomes in a few small case series involving older children [9-14]. Thus far, few studies have investigated the effect of systemic bevacizumab in treating recalcitrant RRP in younger children. In our case report, we describe our successful experience in treating aggressive RRP with systemic bevacizumab in two young children.
Case 1
Following Institutional Review Board approval from Children’s Hospital of Orange County (CHOC), two young children with aggressive RRP treated with systemic bevacizumab were retrospectively reviewed. The first patient was a 2-year-old boy who presented to the Pediatric Otolaryngology clinic with increasing stridor and activity intolerance. In-office flexible laryngoscopy revealed near complete airway obstruction secondary to RRP. The patient was taken urgently to the operating room for initial debridement (Figure 1A). Subsequently, the patient underwent 12 additional microdebridement surgeries with progressively decreasing intervals between surgery over the course of the following 18 months. His voice developed a coarse and raspy quality as a result of repeated instrumentation and microdebridements. On laryngoscopy each time, he was noted to have a near complete obstruction of the vocal cords despite aggressive surgical therapy. The risks and benefits of initiating systemic bevacizumab off-label for the management of RRP were discussed in detail, and the parents agreed to proceed with the novel treatment strategy. The patient was started on an infusion of systemic bevacizumab at a dose of 10 mg/kg every 3 weeks. A multidisciplinary team of pediatric otolaryngologists and oncologists managed the care of the patient, closely monitoring for side effects. Systemic bevacizumab therapy resulted in immediate symptomatic improvement. Laryngoscopy performed 2 weeks after the initiation of therapy demonstrated complete response with no evidence of disease (Figure 1B). The infusion interval was increased to 4 weeks after 7 months of treatment. After 9 months, the dosage interval was increased to a maintenance interval of 6 weeks. Currently, he is 16 months post initial bevacizumab infusion and has received a total of 15 infusions with no complications or side effects as a result of therapy. He has thus far achieved complete response without needing further operative intervention. Disease surveillance has continued with in-office laryngoscopy.
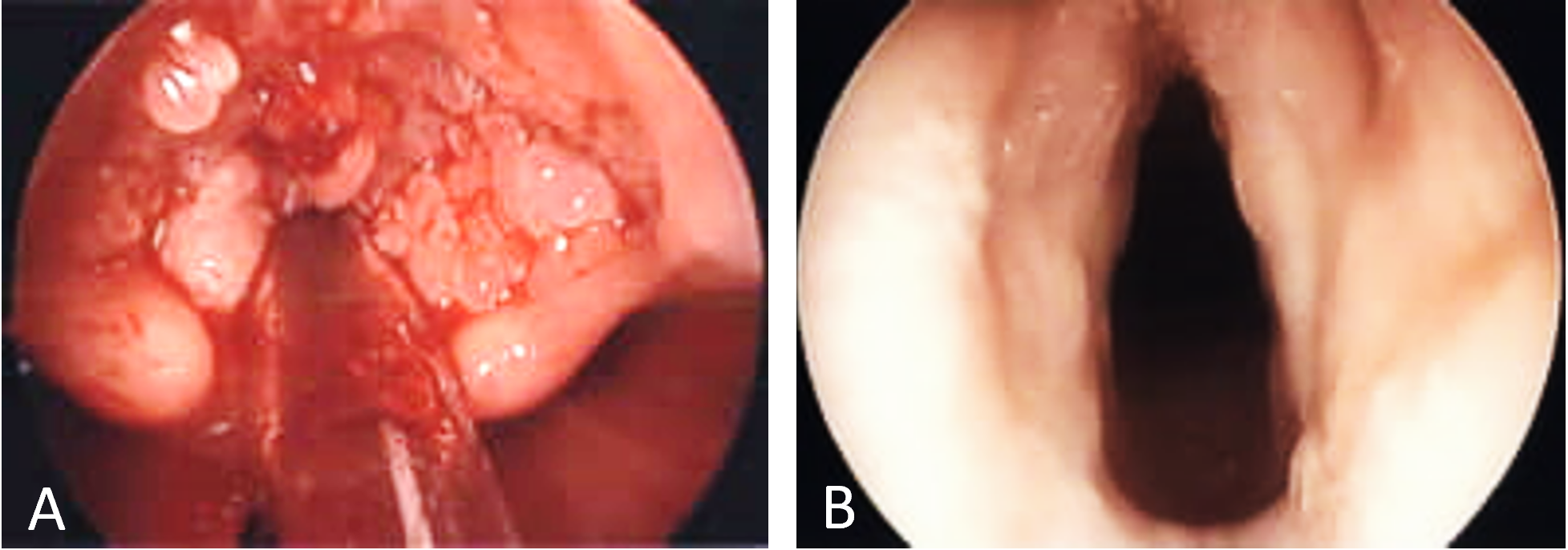
Figure 1. Laryngoscopic findings in case 1. (A) Initial intraoperative laryngoscopy demonstrating papillomatous growth obstructing the vocal cords. (B) Two weeks after the first infusion of bevacizumab demonstrating absence of disease.
Case 2
A 5-month-old boy was diagnosed with RRP after initially presenting with severe airway obstruction (Figure 2A). For the next 9 months he was managed with a total of 11 surgical debridements. The interval between microdebridements decreased from 8 weeks to 3 weeks as his symptoms continued to progress. He was also treated with two courses of adjuvant intralesional cidofovir with no improvement. The papillomas had progressed to near occlusion of the vocal cords and subglottis. The decision to begin systemic bevacizumab therapy was made, and the patient was treated with 10 mg/kg infusions every 3 weeks. After a single infusion, the patient was found to have no evidence of disease at three-week follow up laryngoscopy. After 3 months of infusions, the infusion interval was increased to 4 weeks. After 6 months, the infusion interval was increased to 6 weeks. Surveillance in-office laryngoscopy has revealed no evidence of disease and a complete response. At 10 months of follow-up, the patient continues to receive infusions every 6 weeks and remains free of disease without complications or side effects of bevacizumab infusion (Figure 2B).
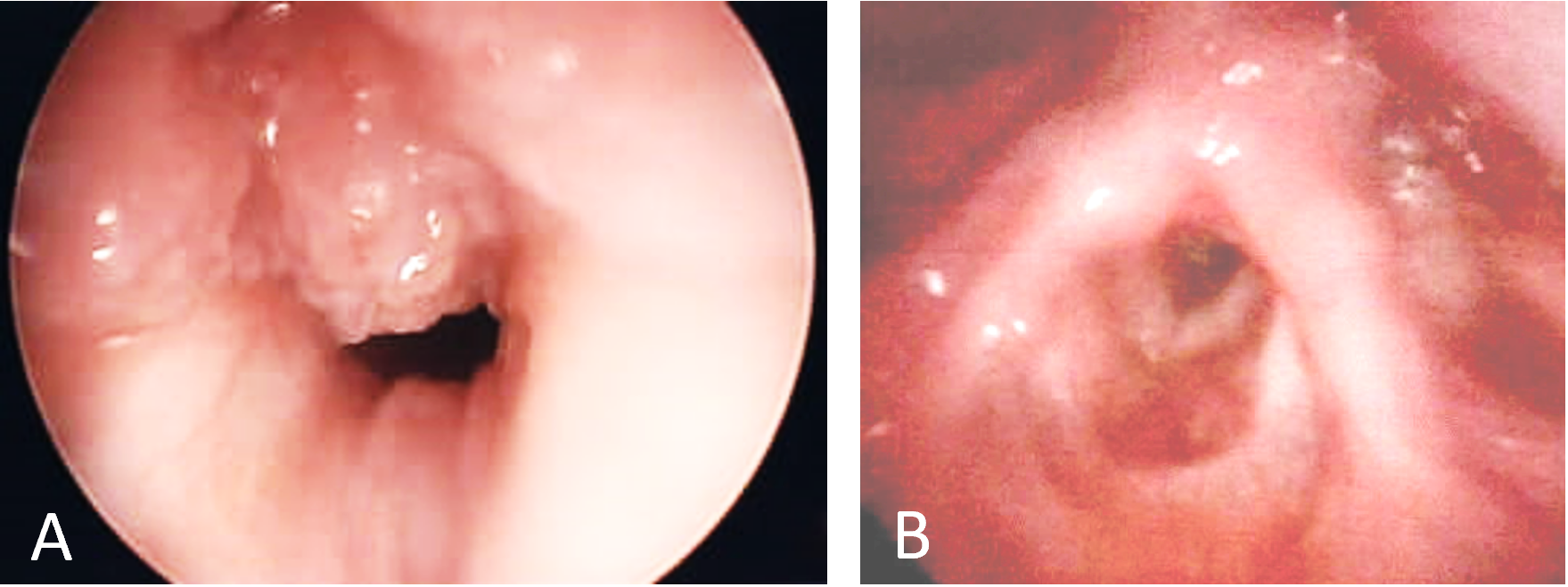
Figure 2. Laryngoscopic findings in case 2. (A) Initial laryngoscopy demonstrating papillomatous growth obstructing the vocal cords. (B) Ten months after initiation of systemic bevacizumab therapy demonstrating absence of disease.
Our case report illustrates the potential dramatic effectiveness of systemic bevacizumab as a treatment strategy for managing aggressive RRP in young children. Both patients in our cohort presented with surgically refractory papillomatosis, requiring frequent microdebridement procedures performed at 3- to 8-week intervals. There was immediate improvement of symptoms and complete response after initiation of therapy as well as no evidence of disease noted on laryngoscopy at 16- and 10-months follow-up, respectively. The treatment has been tolerated well and there have not been any complications or side effects attributed to systemic bevacizumab therapy.
Bevacizumab is a recombinant humanized monoclonal antibody that inhibits angiogenesis by targeting circulating vascular endothelial growth factor A (VEGF-A), preventing it from binding to its receptor [15]. It was first introduced in the U.S. in 2005 and has been used to treat a wide range of cancers such as non-small-cell lung carcinoma, metastatic colorectal cancer, metastatic breast cancer, renal cell carcinoma, ovarian cancer, and glioblastoma multiforme [16]. In 2009, Nagel et al. first demonstrated successful treatment of aggressive RRP with systemic bevacizumab in an adult. Since then, several studies have been published exploring the effects of adjuvant bevacizumab therapy administered both systemically and intralesionally [17]. Bevacizumab treats RRP by blocking the formation of blood vessels that supply rapidly growing papillomas, thus preventing obstructive tumor formation throughout the aerodigestive tract [9].
Systemic bevacizumab can be especially useful in young children who typically present with more aggressive disease compared with older children and adults. RRP presents predominantly in two age groups classified as juvenile onset (<12 years old) and adult onset (ages 20-40). Juvenile onset RRP presents with a greater extent of disease, requires a higher frequency of surgeries, and carries a worse prognosis compared with adult onset [18]. Furthermore, the younger the age at presentation, the more detrimental the clinical course [19]. Hence, bevacizumab should be strongly considered in younger patients who may benefit from early intervention.
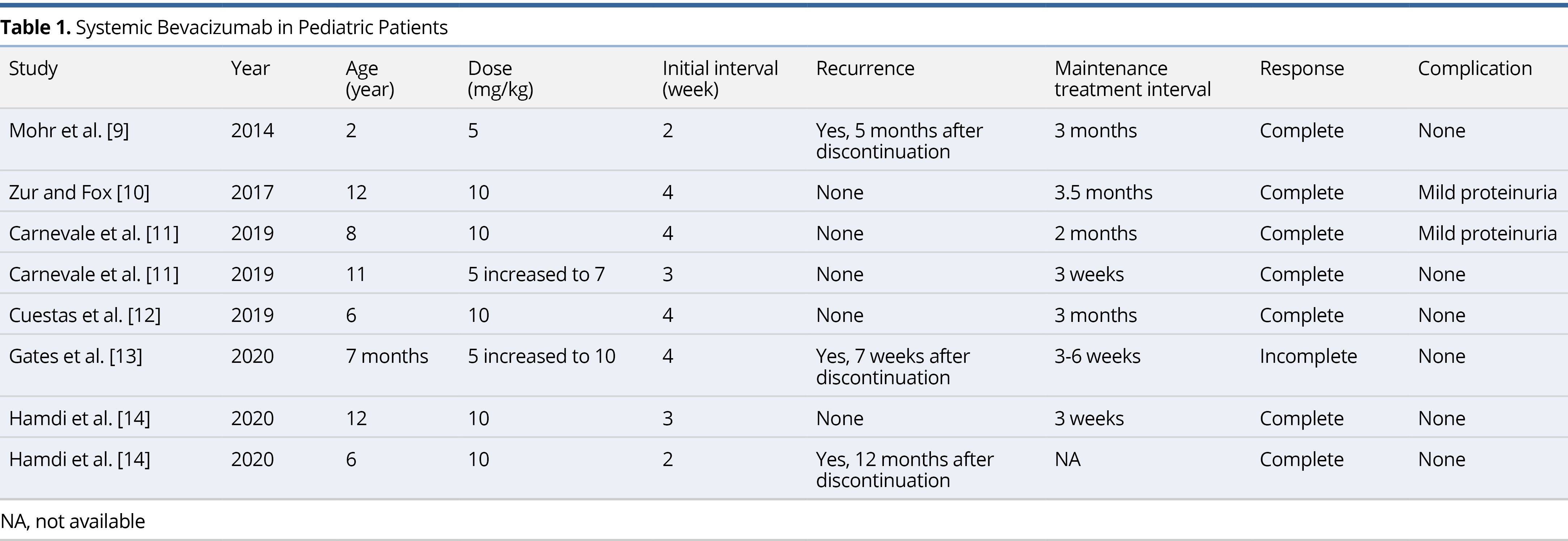
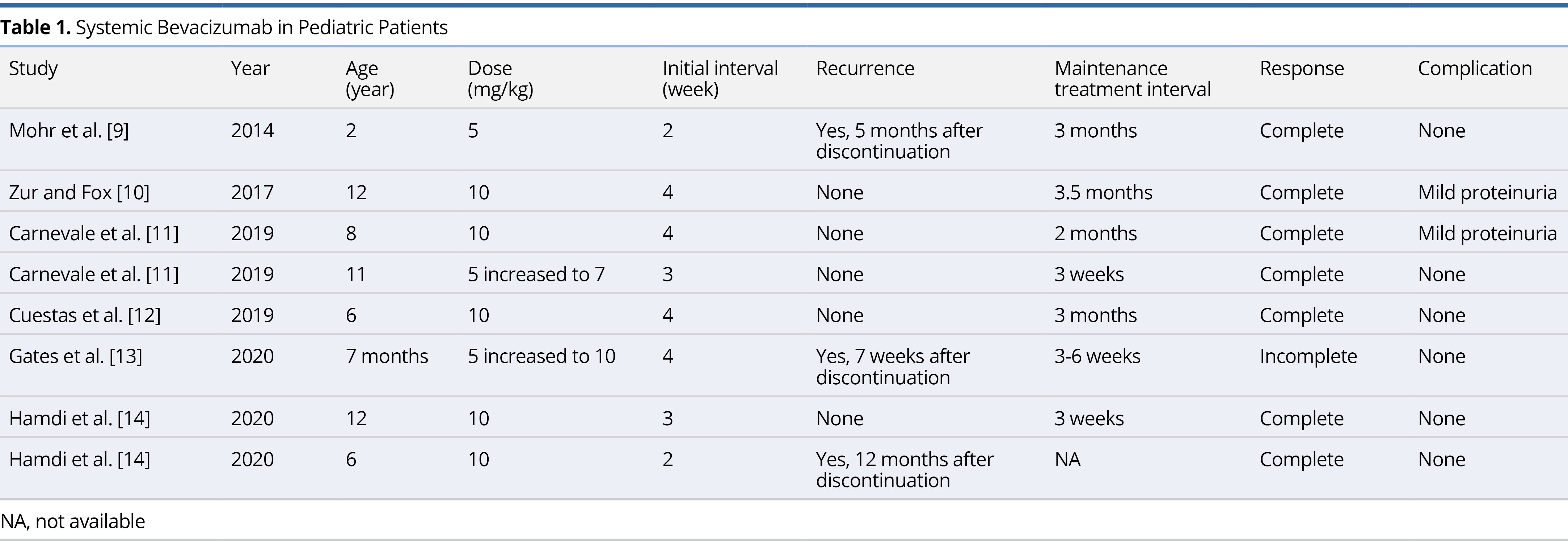
Mohr et al. was the first to initiate therapy on a child in 2014. Since then, a total of 6 case reports and case series have been published on the use of systemic bevacizumab for the treatment of RRP in children (Table 1) [9-14]. The average age of the patients in these reports was 7.2 ± 4.4 years (range 7 months to 12 years). Five patients (62.5%) were started on the 10 mg/kg dose originally described in the adult literature. Two patients were started on a lower dose of 5 mg/kg before increasing to 7 mg/kg and 10 mg/kg, respectively. One patient remained on a 5 mg/kg dose, however, infusions were delivered at a more frequent interval of every 2 weeks. The average initial interval for infusions was 3.25 weeks and the average maintenance interval was 8.07 weeks. There has been a total of 8 pediatric patients treated with systemic bevacizumab, all of whom experienced improvement in symptoms. Three patients (37.5%) experienced recurrence of disease, however, in all three cases therapy was discontinued. There was only one article describing failed long-term clinical improvement with the use of systemic bevacizumab therapy [13]. The case involved a 7-month-old male patient with severe RRP requiring tracheostomy placement and serial bronchoscopy with microdebrider excision. Within 10 months of treatment, the patient displayed reduced or lack of growth at most locations and no new sites of growth. The decision to discontinue treatment was made, and after 7 weeks, the patient was found to have significant regrowth of lesions. All 5 patients described in the literature who received continuous bevacizumab experienced a complete response to therapy, defined as complete resolution of symptoms without evidence of disease on follow up laryngoscopy. Two patients (25%) experienced mild proteinuria as a side effect of bevacizumab, but these findings were transient and had no impact on delivery of therapy. Similarly, the patients in our current report experienced complete response without any side effects attributable to bevacizumab.
The current mainstay of treatment for RRP is surgery; however, with aggressive forms of disease the effect of surgery may be short-lived and may lead to long-term complications. Vocal cord scarring as a result of trauma during surgery frequently leads to dysphonia [20, 21]. Furthermore, patients undergoing multiple surgeries are at risk of developing more serious complications such as vocal cord synechia and glottic stenosis [22]. Early intervention with infusion therapy may reduce the number of microdebridement surgeries and its associated complications. Another important consideration is the effect of multiple anesthesia events on a young child. The Food and Drug Administration (FDA) has issued a black-box warning that repeated use of general anesthesia in children younger than 3 years may have an adverse effect on brain development [23]. Repeat debridement surgeries also presents a significant financial burden for both the patient and the healthcare system, with the annual cost of treatment estimated to be $57,996 per patient and between $40 million and $123 million to the United States healthcare system [24]. Systemic bevacizumab serves as an effective alternate treatment strategy, obviating the need for multiple debulking surgeries.
One of the major advantages of systemic bevacizumab is its predictability in maintaining airway patency. Patients with aggressive RRP being managed by serial microdebridements will often transition between intervals of airway patency and airway obstruction, without any clear pattern of disease [1]. This uncertainty carries an enormous emotional burden for both young children and their families [25]. Bevacizumab offers an alternative strategy with regular treatment intervals and an absence of symptom recurrence between intervals.
Similarly to adults, bevacizumab is fairly well tolerated in the pediatric population. The main side effects are hypertension and proteinuria, both of which are reversible with discontinuation of the drug [26, 27]. Side effects are not dependent on the dose or the duration of treatment. Although rare, systemic bevacizumab may lead to the development of serious side effects such as bleeding, delayed wound healing, and gastrointestinal perforations [28]. Furthermore, bevacizumab is a relatively new drug and data regarding its long-term adverse effects is limited [25]. Prior to initiating systemic bevacizumab therapy, it is important to provide extensive pre-treatment counseling to parents to inform them of the potential risks of using systemic bevacizumab off-label for the treatment of RRP. Systemic bevacizumab should only be reserved for the most severe cases of RRP that are refractory to surgery, and it is imperative that this be communicated to the parents in order to make an informed decision.
Although no definitive guidelines for managing aggressive RRP exist, there are recommendations in the literature for the use of adjuvant therapy in conjunction with surgery. Criteria include (1) rapid recurrence of papillomas, (2) greater than four surgeries per year, and/or (3) distal multi-site spread of disease [29]. These criteria were developed with less aggressive adjuvant therapies in mind, such as intralesional cidofovir or interferon. We support using systemic bevacizumab therapy in children who present with recurrent airway obstruction requiring operative intervention every 4-6 weeks for a duration of at least 6 months. Age should not be a factor in determining candidacy for therapy as young children have benefited greatly from systemic bevacizumab and the drug has been well-tolerated with a mild side-effect profile. We recommend starting infusions of 10 mg/kg at 3-week intervals, and then tapering therapy according to response to treatment. The course of disease has been shown to be highly variable; therefore, infusion intervals must be individualized to the patient. When changing infusion intervals, in-office flexible laryngoscopy can be used for disease surveillance.
Our case report is limited by the number of subjects and its retrospective nature. Despite its limitations, our case report demonstrates that young children with severe RRP can undergo systemic bevacizumab therapy safely with dramatic complete responses. A drawback to this treatment strategy is the need for a port placement, which is invasive, requires maintenance, and puts the patient at risk for port site infection. Nonetheless, in young children with severe airway obstruction as a consequence of RRP, bevacizumab systemic infusion should be considered as an option for adjuvant therapy. Clinical trials with long term follow up will be useful in determining the appropriate indications, dosing, and intervals in order to optimize the treatment protocol.
Our case report suggests that systemic bevacizumab may be a useful adjuvant treatment option to consider for young children with RRP. While serial microdebridements remains the gold standard for treatment of aggressive juvenile RRP, patients requiring more than 4 microdebridements per year can benefit greatly from systemic bevacizumab infusions. This benefit can be most appreciated in younger children who have been shown to exhibit more severe disease and can benefit greatly from early infusion therapy.
Received date: March 31, 2021
Accepted date: October 21, 2021
Published date: November 29, 2021
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2021 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Bruss DM, Berger MH, HaDuong JH, Huoh KC. Improvement in pediatric recurrent respiratory papillomatosis with systemic bevacizumab: Case report and review of the literature. Arch Otorhinolaryngol Head Neck Surg 2021;5(1):4. https://doi.org/10.24983/scitemed.aohns.2021.00148