Various modalities for cancer treatment, including tumor resection surgery and adjuvant chemotherapy, can disrupt the lymphatic drainage in the lower extremities, potentially causing lymphedema. This report details a case of a 79-year-old female patient who underwent a series of procedures including transurethral incision of the ureteral orifice, laparoscopic nephroureterectomy, and adjuvant chemotherapy, as treatment for invasive urothelial carcinoma. Following these treatments, the patient developed symptoms of lower limb edema, later diagnosed as lymphedema via lymphoscintigraphy. The patient's lower extremity lymphedema initially improved following lymphovenous anastomosis. However, the edematous condition recurred after one year. Initially, the patient was presumed to have recurrent lymphedema, a logical assumption given that around 36% of patients necessitate revision of postoperative anastomosis. However, the subsequent diagnosis of deep vein thrombosis, followed by its appropriate treatment, led to an improvement in the lower extremity edema. This case highlights the significant risk of misdiagnosing deep vein thrombosis as recurrent lymphedema in patients who have undergone lymphovenous anastomosis, primarily due to the clinical similarities between the two conditions. Such misdiagnoses can lead to considerable delays in the administration of appropriate treatment for deep vein thrombosis. This study underscores the critical necessity of conducting a comprehensive reassessment in older cancer patients experiencing recurrent leg swelling following lymphovenous anastomosis. It also stresses the importance of accurately differentiating between a recurrence of lymphedema and other potential complications, such as deep vein thrombosis, to ensure the provision of timely and effective treatment.
In the postoperative management of lymphovenous anastomosis (LVA), initially suspecting lymphedema recurrence as the primary cause of re-emerging symptoms is a logical and methodologically sound practice. However, this standard approach may inadvertently overshadow other diagnoses, especially in cancer patients who are undergoing various treatments. A key diagnostic challenge arises in distinguishing between recurrent lymphedema and lower extremity swelling indicative of deep vein thrombosis (DVT). The latter may be a side effect of immunotherapeutic regimens commonly used in cancer treatment [1,2]. The clinical presentation of both conditions is often similar, with swelling being a common symptom, leading to frequent misdiagnoses.
Empirical evidence suggests that a considerable number of patients receiving cancer immunotherapy, about one-fourth, experience venous thromboembolism, which correlates with decreased overall survival rates [1]. Additionally, case studies have shown that patients with urothelial carcinoma can develop thrombotic microangiopathy in association with pembrolizumab therapy post-immunotherapy [2]. This evidence underscores the importance of considering a patient's immunotherapy history when diagnosing lower extremity edema, highlighting the potential for misdiagnoses that could delay critical DVT interventions.
This report presents a case study of an elderly patient post-LVA surgery, illustrating the complexities in post-surgical care. We explore the diagnostic process, interventional strategies, and the necessity for nuanced decision-making, especially in managing geriatric patients. This case study aims to illuminate the intricacies of postoperative care in oncologic surgery and emphasizes the need for tailored patient management strategies in the context of complex surgical histories.
In December 2019, a 79-year-old female patient presented with hematuria. Her medical history was notable for hypertension, diabetes (managed with vildagliptin), and hepatitis C. An ultrasound, revealing hydronephrosis in her left kidney, led to further investigations including abdominal and pelvic computed tomography and a retrograde pyelogram, primarily to explore the possibility of renal stones. However, subsequent ureterorenoscopic stone manipulation resulted in a pathology report diagnosing left invasive urothelial carcinoma.
In February 2020, the patient underwent a dual surgical procedure consisting of a transurethral incision of the ureteral orifice and laparoscopic nephroterectomy. The pathological examination post-surgery confirmed high-grade invasive urothelial carcinoma extending from the ureter to the renal pelvis, classified as pathologic grade T3. Following surgery, in March 2020, she commenced an adjuvant chemotherapy regimen, comprising eight cycles of gemcitabine and carboplatin.
In March 2021, the patient sought medical attention for persistent bilateral lower limb edema, which had been ongoing for eight months and was more pronounced in her left leg. Despite the lack of DVT findings in the abdomen and pelvis computed tomography scans, her history of stage III invasive urothelial carcinoma, along with previous surgery and chemotherapy, necessitated further investigation. A lymphoscintigraphy examination was conducted, revealing partial mild lymphatic obstructions in both lower extremities (right P-1, left P-1, according to the Taiwan Lymphoscintigraphy Staging [3]). Clinically, both legs were categorized as stage II lymphedema, based on the International Society of Lymphedema staging [4].
After four months of conservative treatment, including compression garments, with no significant improvement, the patient underwent LVA on her left leg. A total of six side-to-end LVAs were performed, involving three in the left dorsal foot, one in the left lower leg, one in the left thigh, and one in the left lower abdomen (Figure 1). A compression garment was applied immediately after the operation. The postoperative course proceeded smoothly, and the patient was given empirical antibiotic cefazoline and pain control for three days. She was discharged three days post-surgery and scheduled for regular follow-ups in the outpatient clinic. At the follow-up on postoperative day 8, there was a noticeable improvement in edema in both legs (left leg reduced from 33.3 ± 10.7 cm to 26.2 ± 8.5 cm, right leg from 29.6 ± 7.9 cm to 27.5 ± 7.8 cm).
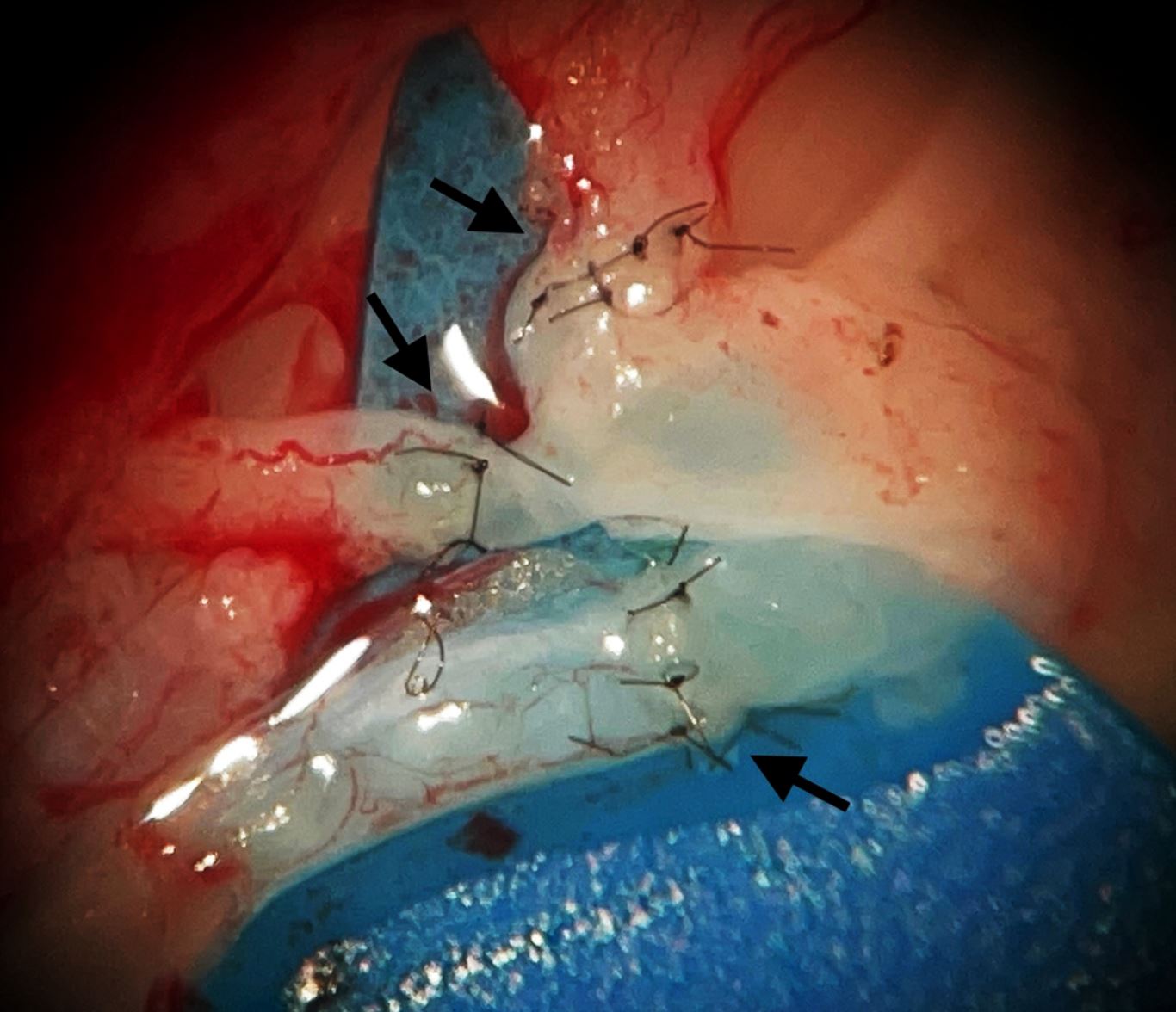
Figure 1. Three lymphovenous anastomoses (indicated by black arrows) are present in the dorsal foot wound during lymphovenous anastomosis surgery.
In March 2022, seven months post-LVA surgery, the patient began an 11-cycle pembrolizumab treatment due to tumor progression. Concurrently, she experienced recurrent swelling in her left leg, persisting for eight months before she sought help at the emergency department in November 2022. Upon examination, her left leg exhibited a swollen appearance, mild reddish skin, and discharge formation. Vascular sonography of the left lower limb revealed a partial thrombus in the left common femoral vein. Initially, Edoxaban was administered, temporarily alleviating the symptoms.
However, two months later, the patient returned to the emergency department with worsened pain and swelling in her leg. She was diagnosed with recurrent DVT and leg cellulitis. Initial vascular ultrasonography and computed tomography angiography identified a mural thrombus in the left common femoral vein, with a patent popliteal and calf vein. Subsequently, percutaneous transluminal angiography revealed bilateral common iliac vein occlusion. The patient underwent stent placement, which resulted in a reduction of the swelling in both legs postoperatively (Figure 2).
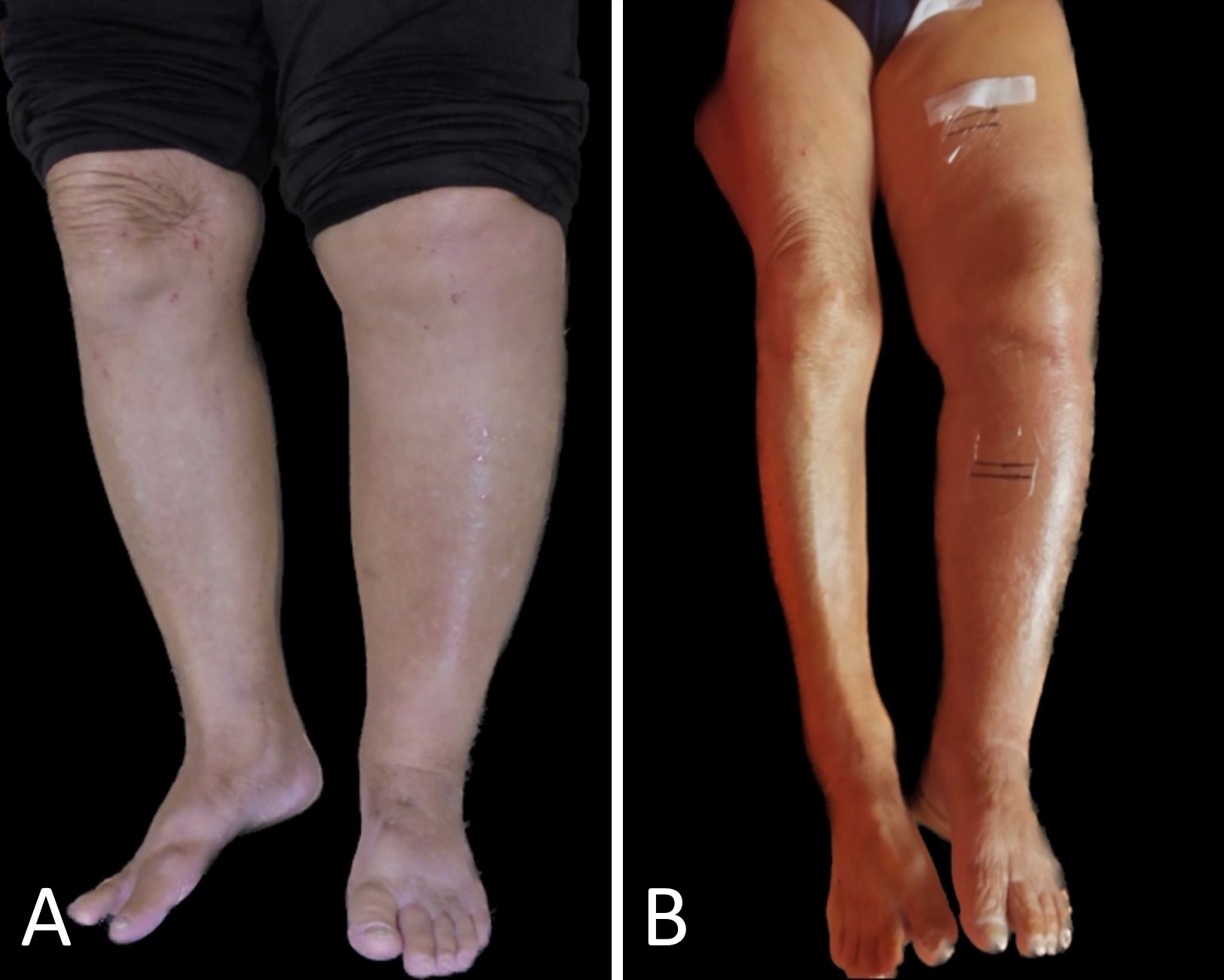
Figure 2. Comparative images showing the condition before (panel A) and after (panel B) treatment of deep vein thrombosis in the bilateral common iliac femoral veins. Post-treatment, there is a noticeable reduction in swelling in both legs.
LVA has been established as an effective intervention for improving lower limb lymphedema, as evidenced in recent studies [5,6]. However, approximately 36% of patients require postoperative anastomosis revision [7]. Therefore, it is both reasonable and sensible to first consider lymphedema recurrence as the main concern when patients, who have undergone LVA, exhibit returning symptoms. Nevertheless, this approach entails a significant risk: Owing to their clinical similarities, patients who have undergone LVA and subsequently develop DVT are at a high risk of misdiagnosis as cases of recurrent lymphedema. Such misdiagnoses can lead to significant delays in the appropriate treatment for DVT. This study aims to underscore the critical need for thorough reassessment in older cancer patients who experience recurrent leg swelling after undergoing LVA. It highlights the importance of distinguishing between lymphedema recurrence and other potential complications like DVT to ensure timely and accurate treatment.
The primary complications associated with LVA include failed anastomosis, venous reflux, and cellulitis [7]. In clinical practice, when encountering patients with lymphedema who have previously undergone LVA and present with swollen feet, the initial diagnostic considerations typically focus on lymphedema recurrence and infection. Conversely, DVT is not commonly considered a primary diagnosis due to its relative infrequency as a complication of LVA.
However, this case presented additional risk factors that increased the patient's susceptibility to thrombosis-related edema. Notably, age was a significant risk factor for this 79-year-old patient [8]. Research indicates a substantial rise in DVT incidence among patients aged 70 and above [9]. Additionally, the patient's decreased mobility, a consequence of leg swelling, might have further heightened her risk of developing DVT [10]. This case serves as a reminder to clinicians that even when a patient has a well-documented history of LVA, commonly associated with leg edema, a thorough reassessment of the vascular system is essential to ensure an accurate and comprehensive diagnosis of thrombosis-related edema.
Upon reviewing the patient’s medical history, we observed a significant overlap between the onset of foot swelling from DVT and her immunotherapy period with pembrolizumab. This coincidence has led us to consider the possibility that the immunotherapy may have contributed to the development of DVT. Notably, among patients undergoing cancer immunotherapy, nearly one-fourth experience venous thromboembolism, a condition that has been associated with a decrease in overall survival rates [1]. Additionally, a specific case report highlighted that patients with urothelial cancer developed pembrolizumab-associated thrombotic microangiopathy following immunotherapy [2]. These findings collectively indicate that a history of immunotherapy in patients may be a critical factor in the development of symptoms, such as swollen lower extremities.
Complex medical cases often involve patients suffering from both venous edema and lymphedema, as seen in phlebolymphedema. Phlebolymphedema, characterized by swelling due to a combination of chronic venous insufficiency and lymphatic insufficiency, is the most prevalent form of lymphedema in the Western world [11]. Chronic venous insufficiency can develop from venous valve inefficiency, blockages, or a combination of these factors. Furthermore, dysfunction in the muscle pump, particularly in the calf muscles, can exacerbate these conditions [12]. Therefore, it is crucial to maintain vigilance for chronic venous insufficiency both before and after treating lymphedema. Key risk factors for chronic venous insufficiency include age, a family history of venous disease, indicators of ligamentous laxity (e.g., history of hernia surgery, flat feet), body weight, level of physical activity, and smoking [13]. Recognizing and addressing these risk factors are essential steps in the comprehensive management of patients with lymphedema or phlebolymphedema.
The key takeaway from this case is the critical importance of a comprehensive and systematic assessment in patients with complex medical histories. Despite the patient's history suggesting lymphedema recurrence as the primary cause of her foot swelling, the necessity to conduct a thorough evaluation of her venous and lymphatic systems, infection status, and cancer treatment history is paramount. This holistic approach is essential to ensure accurate diagnosis and effective management of the patient's condition. It highlights the need to consider the complexity of the patient’s medical background and the possibility of overlapping symptoms from various conditions. Such an approach is vital in avoiding misdiagnosis and in providing the most effective treatment strategy tailored to the patient's unique medical needs.
In cases of recurrent edema in elderly cancer patients who have previously undergone LVA surgery for lymphedema, it is crucial to consider and rule out phlebolymphedema and DVT as possible causes. To achieve a comprehensive diagnosis, additional assessments like vascular sonography or computed tomography angiography should be conducted. These diagnostic tools can help exclude other potential etiologies and ensure that the patient receives the most appropriate and effective treatment. This approach is vital for a holistic patient care strategy, particularly given the complex interplay of factors in such patients.
Received date: November 28, 2023
Accepted date: January 09, 2024
Published date: January 17, 2024
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2024 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
Authors report a case of lower extremity lymphedema treated by LVA that preoperatively mapped not only lymphatic vessels by PDE, but also veins and venules using Veinsite™ .
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
ICG lymphography is an invaluable tool in lymphedema management. Both immediate and delayed scans are needed when performing the study. The delayed scan needs to be performed at the time of the lymphographic plateau to appreciate the full extent of the pathology. Using a recumbent cross trainer, the lymphographic plateau can be achieved in 15 minutes following ICG injection. We have found this exercise enhanced ICG lymphography protocol worthwhile of adoption by high volume lymphedema centers to raise diagnostic accuracy and efficiency.
In this study, the researchers analyze the intricate interplay among various cancer treatment methods, the emergence of lymphedema, and the incidence of deep vein thrombosis (DVT). The patient in focus initially received treatment for invasive urothelial carcinoma, undergoing procedures such as transurethral incision of the ureteral orifice, laparoscopic nephroureterectomy, and adjuvant chemotherapy. Following these treatments, she developed lower limb edema, which was later diagnosed as lymphedema through lymphoscintigraphy. Despite temporary alleviation through lymphovenous anastomosis (LVA), the edema resurfaced after a year. A significant development occurred when the patient was diagnosed with DVT, for which she received effective treatment, leading to the resolution of the lower extremity edema. This case highlights the complex relationship between cancer-related lymphedema and lower extremity edema, accentuating the necessity to consider additional factors such as age, cancer history, and sedentary lifestyle. It also alerts healthcare professionals to the possibility of DVT as a contributing factor, especially in cases of recurring edema following LVA. The report merits publication in an academic journal due to its clinical importance and educational contribution. Nevertheless, certain aspects warrant consideration prior to its publication.
The case report focuses on an 80-year-old woman who presented with swelling in both lower limbs, notably more severe in her left leg. Her medical history included a high-grade invasive urothelial carcinoma in her left ureter and renal pelvis, treated through surgical and chemotherapeutic interventions. Despite these treatments, she developed progressive swelling in her lower extremities, which was later identified as lymphedema. She underwent lymphovenous anastomosis (LVA) surgery for this condition. However, the swelling recurred, leading to an emergency department visit where she was diagnosed with deep vein thrombosis (DVT). Subsequent treatment for DVT and stent placement helped alleviate the leg swelling. This case report underscores the diagnostic and management challenges of lower extremity edema in such scenarios. The report holds significant value for publication, given its clinical importance and educational contributions. Nonetheless, it is essential to address certain specific concerns before deeming it ready for publication.
This case report presents an insightful analysis of recurrent edema due to deep vein thrombosis (DVT) in a patient who underwent lymphovenous anastomosis (LVA) for cancer-related lymphedema. The patient, an elderly individual with a history of invasive urothelial carcinoma, developed bilateral lower limb lymphedema following cancer treatment. Post-LVA, there was an initial improvement in lymphedema, but the patient subsequently experienced a relapse, which was diagnosed as DVT. This case underscores the importance of considering DVT in patients with recurrent edema after LVA, particularly in elderly patients with risk factors such as a sedentary lifestyle. It also emphasizes the need for comprehensive vascular assessments in similar cases and suggests the inclusion of routine venous ultrasound for effective management. The case report is very informative, especially regarding the patient's medical progression. However, to enhance its suitability for publication, I would like to request additional clarification on several points.
Chen YK, Tung YC, Huang PS, Lin YS. Management of recurrent leg swelling in an elderly patient with invasive urothelial carcinoma: Examining the challenges of post-lymphovenous anastomosis in treating cancer-related lymphedema. Int Microsurg J 2024;8(1):1. https://doi.org/10.24983/scitemed.imj.2024.00179