Background: Lymphaticovenular anastomosis (LVA) is an established supermicrosurgical treatment of the lymphedema. However, success rates vary, possibly related to the variation in patient selection, surgical technique, and postoperative care. One of the controversies on postoperative care is whether to apply limb compression. We set out to assess the effect of external limb compression on the LVA.
Methods: Following each of the anastomoses of the LVA procedure, the flow across the anastomosis was immediately assessed. A “washout” sign was defined as observing the favorable ante grade, lymph-to-vein flow, whereas a “backflow” sign was defined as observing the unfavorable retrograde, vein-to-lymph flow. After the initial flow pattern was recorded, bandage compression was applied to the leg and the changes to the flow pattern were recorded. Patients were tracked with lymphedema indices as well as lymphedema quality of life (LYMQOL) assessment system at preoperative, within the 3rd and 6th month visits.
Results: Five patients were included in the study. 42 LVAs were constructed - 26 with the standard, and 16 via the octopus technique. Initially, 25 (60%) demonstrated “washout”, with the remaining 17 (40%) showing “backflow”. After compression was applied, those entire initially demonstrating washout” maintained the “washout” pattern, while 16 of 17, or 94%, that initially demonstrated “backflow” converted to “washout”. In the follow up, all patients had statistically significant edema reduction based on lower extremity lymphedema indices (P = 0.0009) and relief of symptoms based on the LYMQOL assessment (P = 0.0006).
Conclusion: Postoperative compression following LVA does not harm the anastomoses created, and can augment the lymphatic flow and convert unfavorable retrograde flow to favorable ante grade flow.
Video Abstract
Lymphaticovenular anastomosis (LVA) is a minimally invasive, supermicrosurgical alternative to vascularized lymph node transfer (VLNT) for lymphedema reconstruction [1-3]. The procedure is conceptually simple, and it involves making lymph-to-vein connections via small skin incisions. In contrast to VLNT [4,5], the procedure does not involve harvesting lymphatic tissue and is therefore free of the risk of causing donor-site lymphedema. While its minimally invasive nature is appealing, its reported outcome is disappointingly inconsistent [6-8]. The inconsistency in outcome is likely related to the differences in surgical technique and peri-operative care. The application of postoperative limb compression is one of these controversial differences. Intuitively, limb compression following the LVA procedure may mechanically narrow these minuscule anastomoses, causing anastomotic failure. In standard microsurgery, pressure avoidance at the anastomotic site is an unchallenged dictum. But does the same hold true for the supermicrosurgical LVA? We conducted a simulation study to answer this question – does limb compression following LVA promote or impair lymph-to-vein drainage?
Patients
Five consecutive patients, four female and one male, with age ranging from 23 years to 69 years; undergoing LVA for limb lymphedema, were included in the study (Table 1). All patients had previously failed complex decongestive lymphedema therapy and were referred by our lymphedema therapists for evaluation for surgical reconstruction. All had lower extremity lymphedema. Three had acquired disease and two had primary disease. The severity of disease was staged with Campisi criteria and all had stage II and III diseases.
Lymphedema index is a circumference-based system that takes measurements at five limb levels and references the sum to the patient’s body mass index. LYMQOL is a validated lymphedema-specific quality of life assessment that tracks four condition-specific domains – function, appearance, symptoms, and mood. LEL, lower extremity lymphedema; LYMQOL, lymphedema-specific quality of life assessment; LVA, lymphaticovenular anastomosis; Pre, pre-operative; Post, post-operative.
Study Design
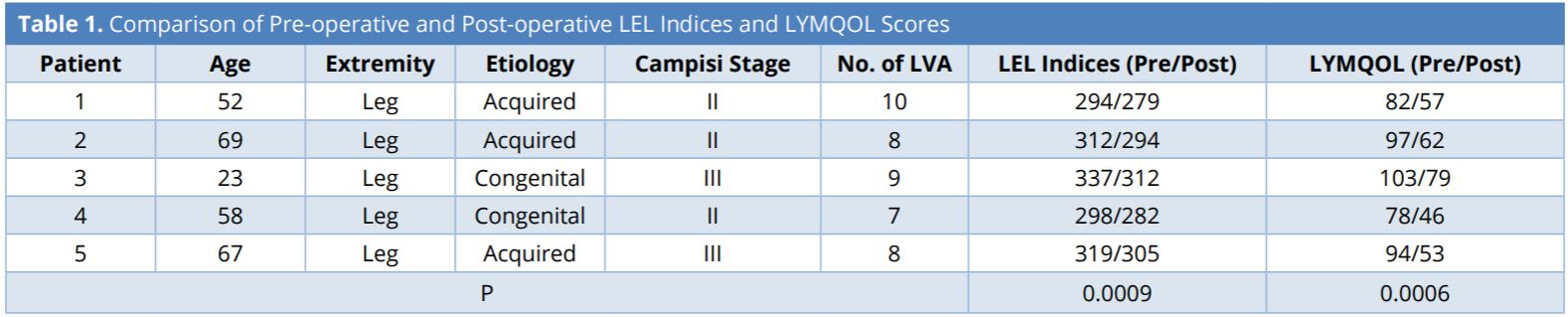
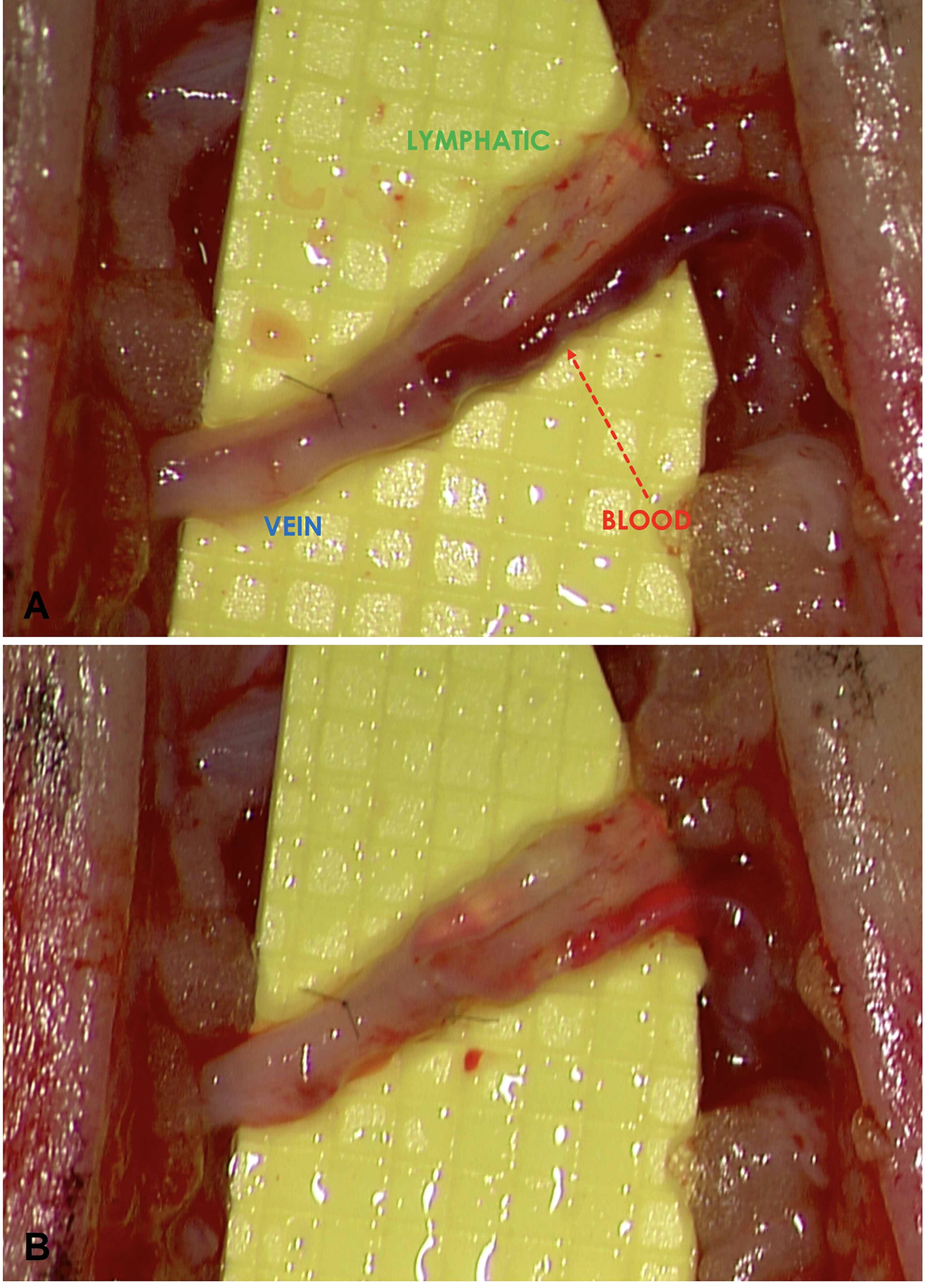
Intraoperatively, immediately following the completion of each of the individual LVAs, the flow pattern across the anastomosis was observed under the surgical microscope. When the lymphatic pressure exceeded the venous pressure, favorable ante grade lymph-to-vein flow occurred and a “washout” sign (Figure 1A) was observed. Conversely, when the pressure gradient was reversed, with the venous pressure exceeding the lymphatic pressure, unfavorable retrograde flow occurred and a “backflow” sign was seen (Figure 1B). Regardless of the initial flow pattern observed, bandage compression was applied to the entire limb to simulate postoperative limb compression (Figure 2). The firmness of compression was determined by the senior author to simulate the compression pressure of 30 – 40 mmHg. Changes to the flow pattern following the bandage compression were observed under the microscope and recorded. Patient evaluation was performed using the circumference-based lower extremity lymphedema (LEL) index system [9] and a lymphedema-specific quality of life assessment (LYMQOL) [10] at preoperative visit; and at the 3rd and 6thmonth postoperative visits. LYMQOL is a condition-specific validated assessment system that tracks postoperative changes in function, appearance, symptoms, and mood. The preoperative and 6-month postoperative LEL and values were compared using paired t-test.
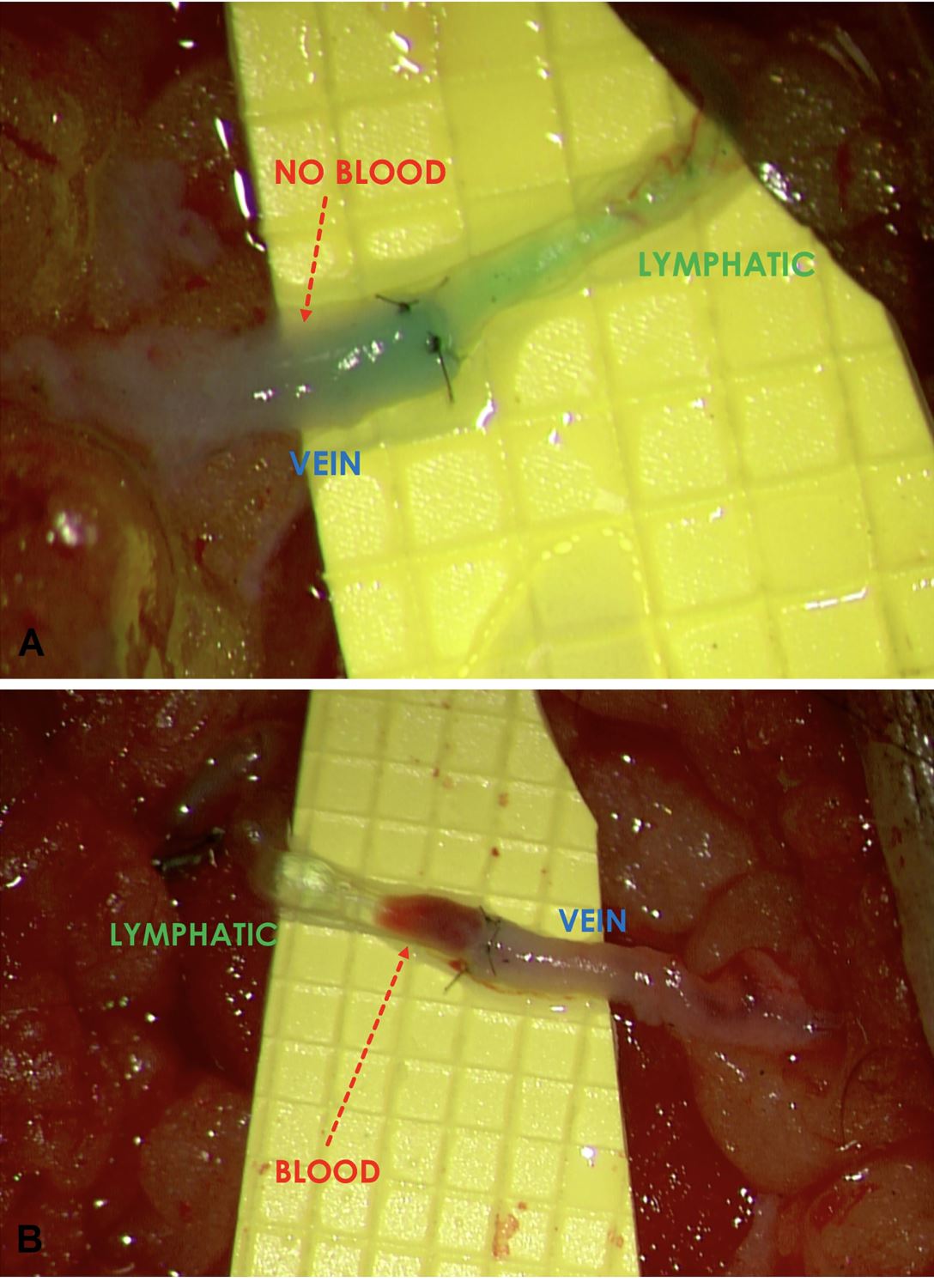
Figure 1. (A) A favorable “washout” sign was observed when the lymphatic pressure exceeded the venous pressure and ante grade flow occurred. (B) Conversely, retrograde flow occurred when the venous pressure exceeded the lymphatic pressure and an unfavorable “backflow” sign was shown.
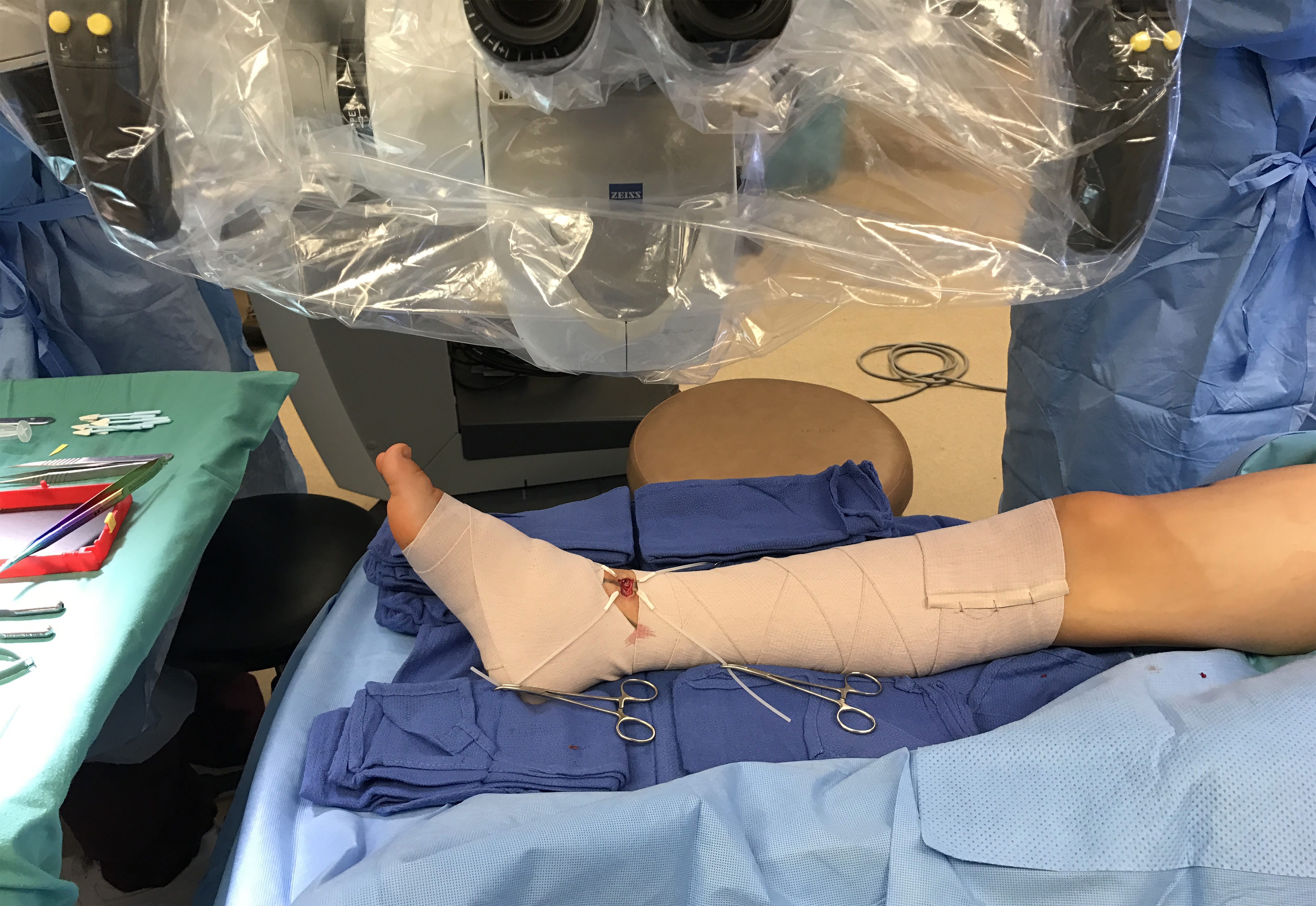
Figure 2. After having documented the initial LVA flow pattern, bandage compression was applied proximal and distal to the incision to simulate the effect of immediate postoperative limb compression.
Surgical Technique
After mapping the lymphatic vessels with indocyanine green lymphography and delineating the superficial venules with an infrared imaging device (VueTek Scientific, Gray, Maine), the incisions were strategically placed at locations where both the lymphatic vessels and venules were present as previously described [8] (Figure 3). 0.05 cc of isofulfan blue (Lymphazurin; United States Surgical Corp., Norwalk, Connecticut) was injected within 2 cm distal to each incision to further facilitate identification of the lymphatic vessels. The LVAs were performed at 25X magnification utilizing a surgical microscope (Pentero 900; Carl Zeiss, Oberkochen, Germany) using specialized supermicrosurgical instruments (EMI Factory, Kitasakugun, Nagano, Japan). Both the standard supermicrosurgical LVA technique described by Koshima and Yamamoto et al. [11-13] and the “octopus” technique [14] were used. When healthy lymphatic vessels and size-matched veins were present, the standard technique was preferentially used. The “octopus” technique was reserved for the challenging situations of 1) lymphatic vessels being severely damaged due to the disease process and 2) number- and/or caliber-mismatch between the lymphatic vessels and the veins. The anastomoses were performed using 12-0 nylon with 50-micrometer needle (S&T, Neuhausen, Switzerland).
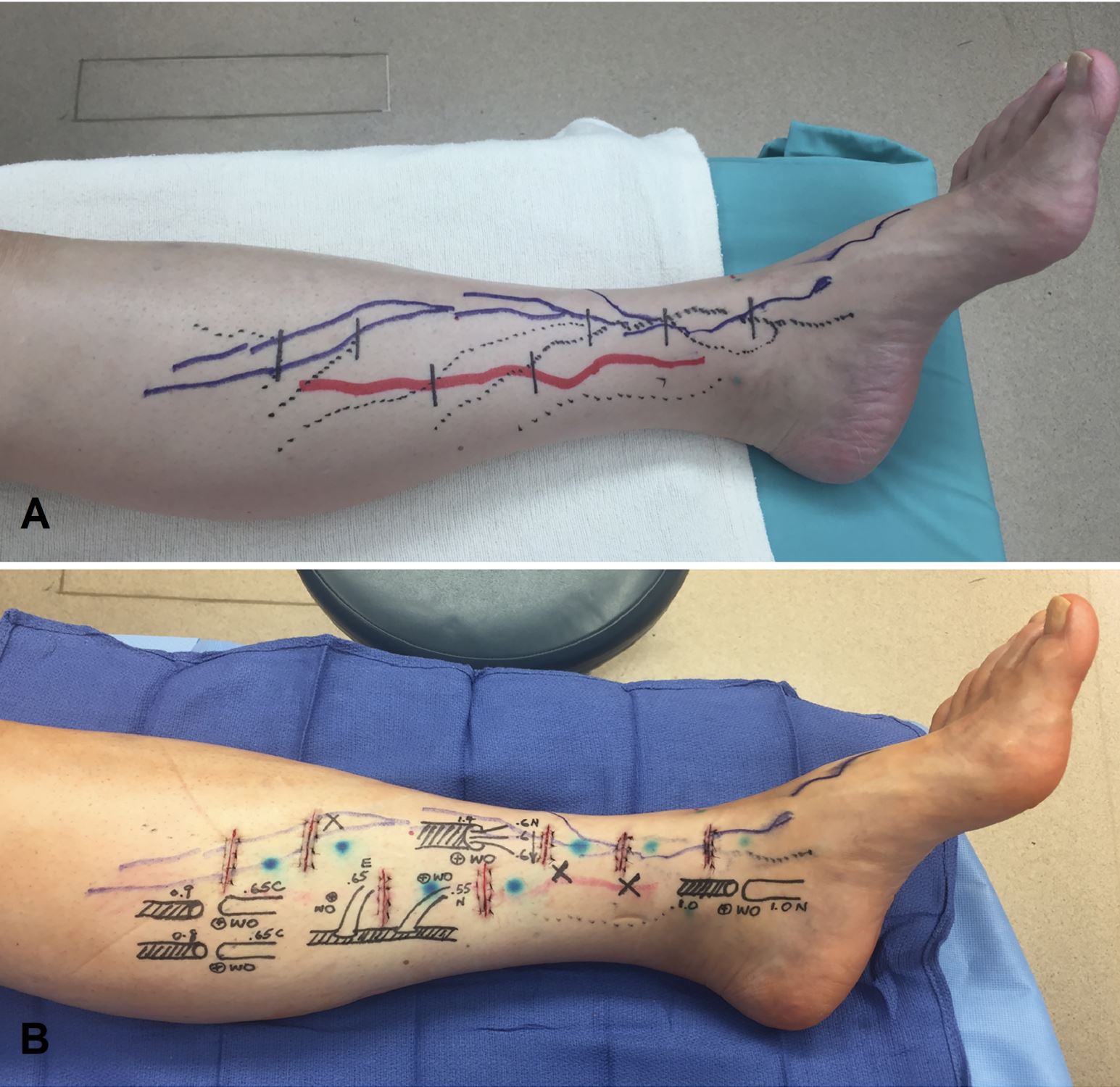
Figure 3. (A) Solid blue and red lines represented lymphatic vessels mapped with indocyanine green lymphography from different injection sites. Dotted lines were superficial venules visualized using the infrared vein finder. Incisions were planned at where the lymphatic vessels and the superficial venules intersected. (B) Postoperative skin markings showing LVAs constructed with various anastomotic techniques.
Postoperative Care
Limb compression was applied immediately following the surgery using the short-stretch bandage. All patients were discharged to home on postoperative day one. Bandage compression continued for 16 hours per day until six weeks postoperatively. At that time, all were transitioned to 30- 40 mmHg pressure. Throughout the 6-month study period, all patients continued to wear their pressure garments for 16 hours per day. Weaning of the pressure garments began at 7 months from the surgery and was not evaluated in this study.
A total of 42 LVAs were created – 26 standard LVAs and 16 “octopus” LVAs. Initially, 25 of the LVAs (60%) demonstrated the favorable “washout” sign and the remaining 17 (40%) demonstrated the unfavorable “backflow” sign. Among these 17 LVAs demonstrating the “backflow” sign, 13 were the “octopus” and 4 were the standard LVAs. Following the application of limb compression, 16 of the 17 LVAs (94%) converted the flow pattern from the unfavorable “backflow” to the favorable “washout” (Figures 4A & 4B). The conversion rates of the “octopus” and the standard LVAs were 100% (13/13) and 75% (3/4), respectively. All 25 LVAs initially demonstrating “washout” maintained the “washout” pattern following the bandage compression. Regardless of the initial flow patterns, post-compression vessel engorgement was observed in the lymphatic vessels (Figure 4B). This finding along with the post-compression flow reversal suggested a compression-induced augmentation of the lymphatic pressure.
All patients demonstrated reduction of limb swelling and relief of symptoms, as demonstrated by statistically significant improvements in the lower extremity lymphedema indices (P = 0.0009) and in the LYMQOL (P = 0.0006) (Table 1). All patients reported their symptoms being notably more responsive to compression. No patient had worsening of lymphedema symptoms during the 6-month study period.
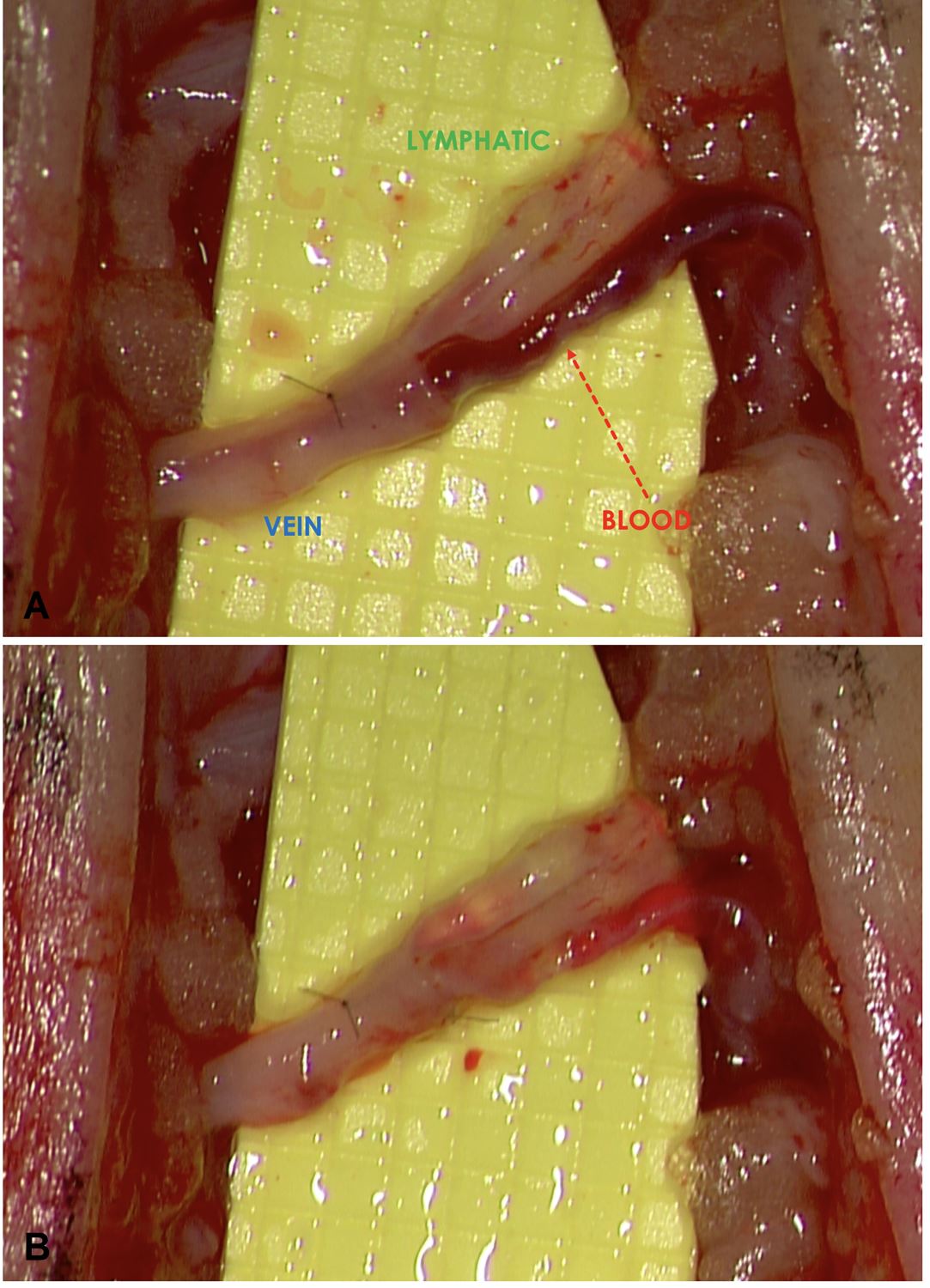
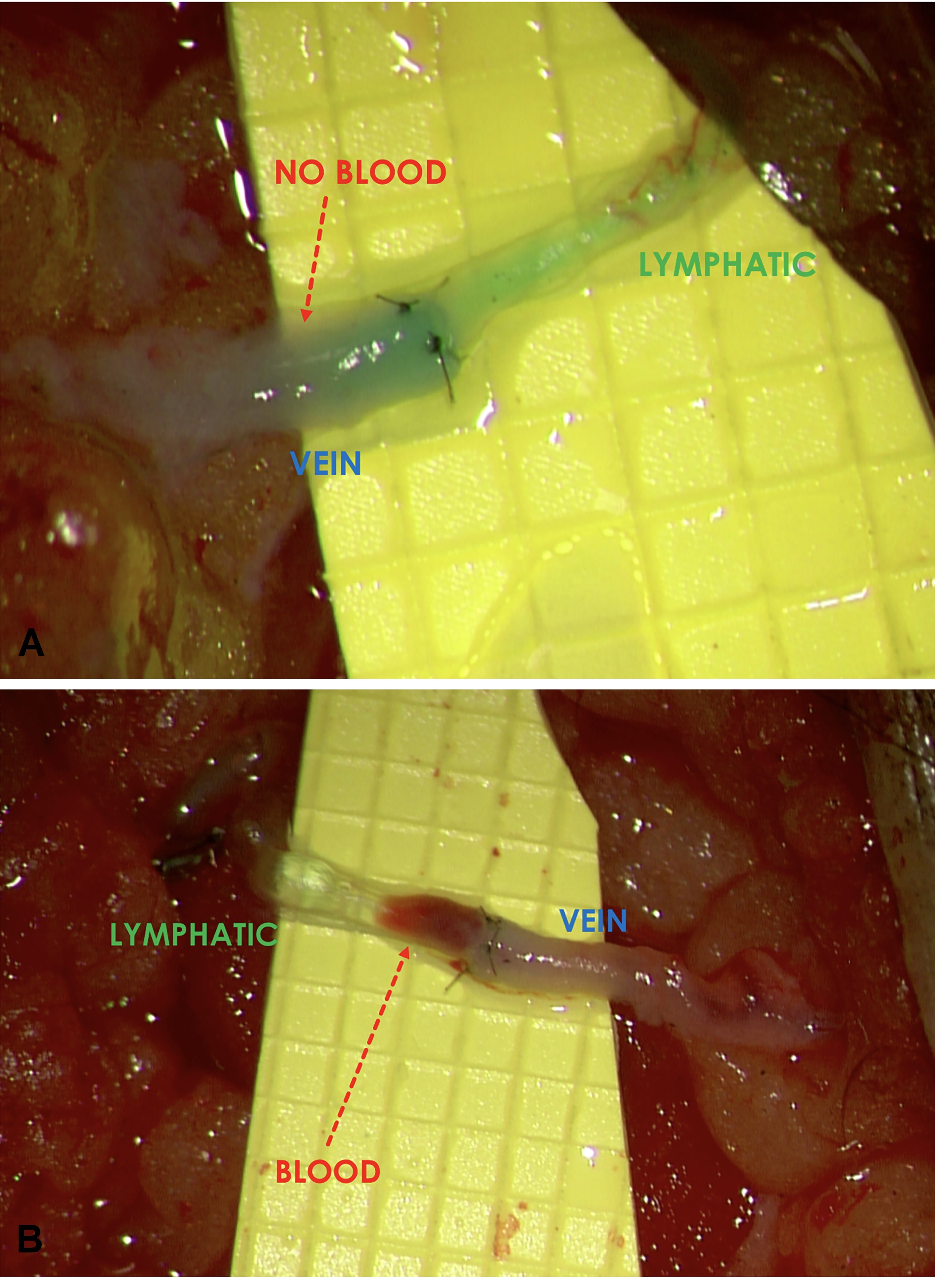
Figure 4. (A) Before compression. A 3 lymphatic vessel-to-1 vein LVA anastomosed using the “octopus” technique showing “backflow” in all 3 lymphatic vessels. The “backflow” sign was particularly prominent in the most inferior lymphatic vessel. (B) After compression. Conversion to “washout” was seen in all 3 of the lymphatic vessels. Note how the blood was “washed out” of all 3 of the lymphatic vessels and the vein. Engorgement of the lymphatic vessels was clearly seen in the top 2 lymphatic vessels.
LVA is a delicate supermicrosurgical procedure. Until now, most studies focused on the intricate technical aspects of the procedure [13-16] and little had been described about postoperative management. As we become more proficient in creating these tiny anastomoses, it is important to start to evaluate other procedural parameters to maximize surgical efficacy. The opinions and practices on postoperative limb compression following LVA vary widely among the supermicrosurgeons. Common practices include no compression [17,18], delayed compression starting few weeks following the surgery [11], and immediate compression [6]. To our knowledge, this is the first simulation study directly evaluating the effects of compression on the LVA.
The findings in this study support immediate postoperative limb compression. When the compression was applied, majority of the unfavorable LVAs with retrograde flow converted to the favorable functioning LVAs with ante grade flow (94%). When left untreated, the refluxed blood in the lymphatic lumen may cause thrombosis and result in anastomotic failure. Interestingly, the limb compression appeared to create a generalized augmentation of the lymph-to-vein pressure gradient, as suggested by visible engorgement of lymphatic vessels (Figure 4B). This phenomenon was seen in all the LVAs, including those already demonstrating ante grade flows prior to compression. This finding along with the high rate of favorable flow conversion suggested functional enhancement of the LVAs with postoperative limb compression.
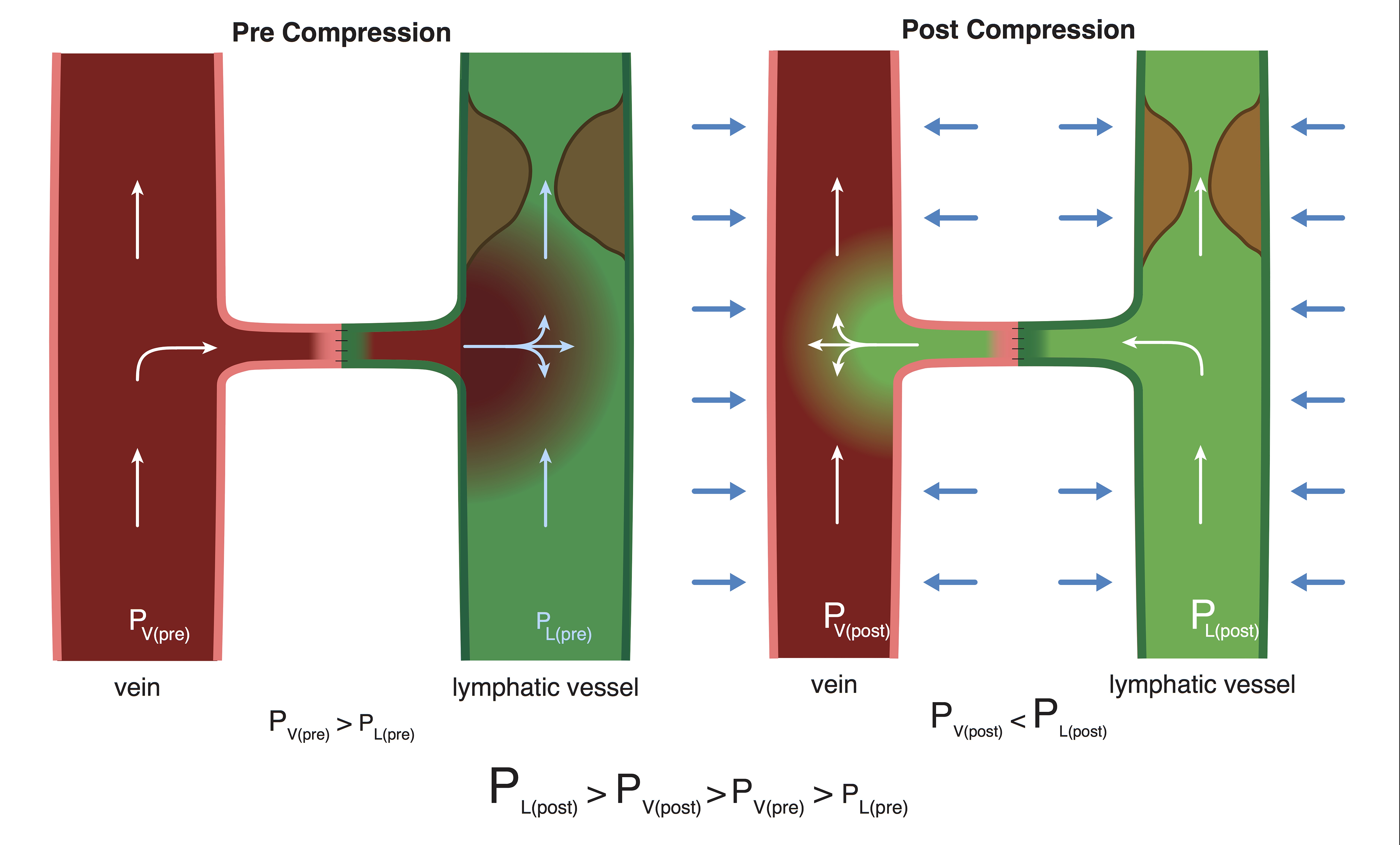
Why would limb compression, which pressurized both the venous and lymphatic systems simultaneously, alter the lymph-vein pressure gradient? We hypothesized that the differential effects on the two systems in response to external pressure may be related to the lymphatic system being a partially obstructed system and the venous system being a non-obstructed, free-flowing system. When both systems were simultaneously pressurized, the partially obstructed system experienced a higher magnitude of pressure increase relative to the open system due to its inability to efficiently decompress (Hai Fu, Department of Physics and Astronomy, University of Iowa, personal communication, December 22, 2016) (Figure 5). In contrast, not having an outflow obstruction, the open venous system could quickly decompress and therefore experienced a lesser pressure increase.
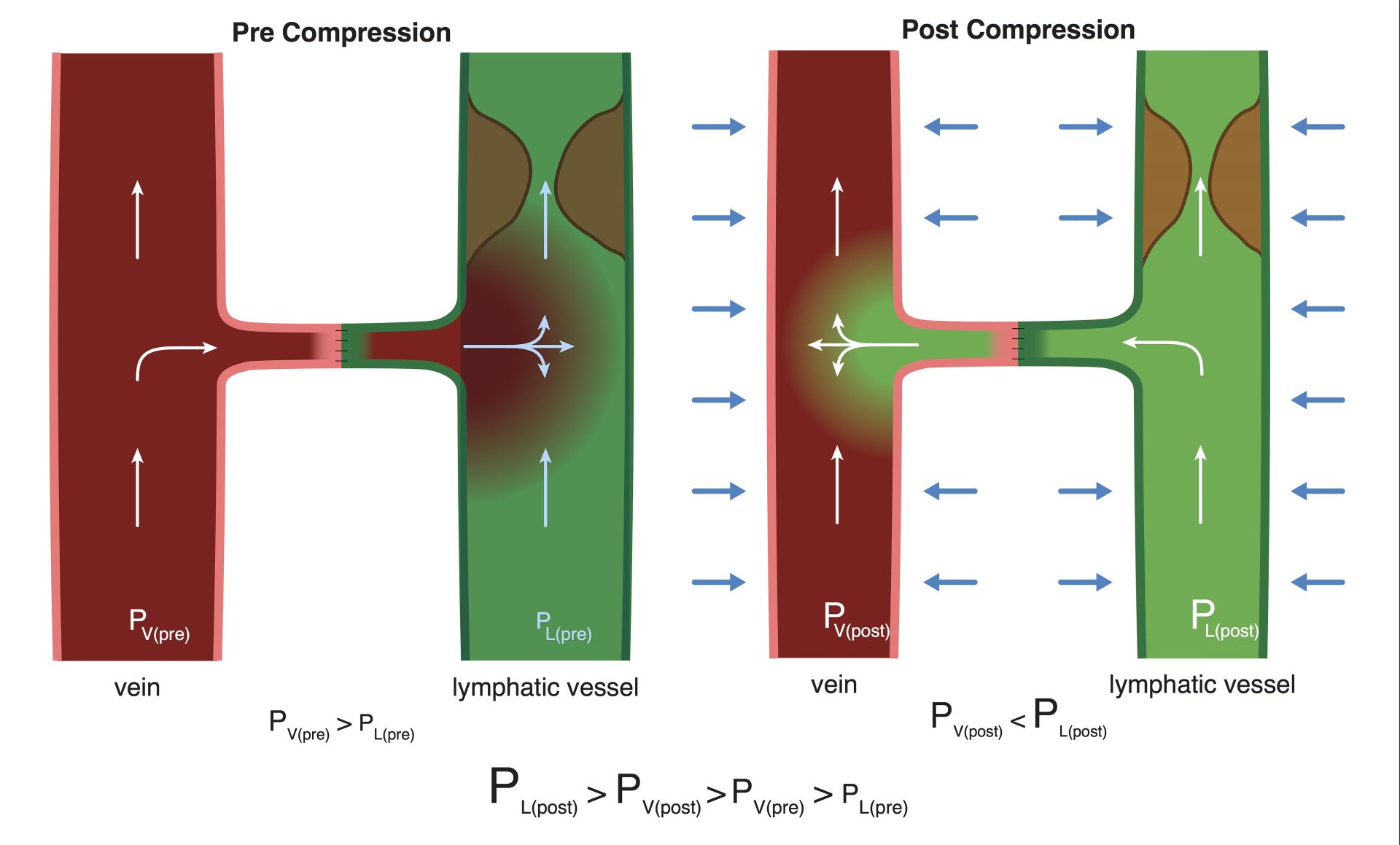
Figure 5. The mechanism of differential pressure changes in the lymphatic and venous systems in response to limb compression. Prior to compression, venous pressure > lymphatic pressure and “backflow” occurred. With compression, the lymphatic system experienced a significantly greater pressure increase due to the presence of outflow obstruction associated with lymphedema, resulting in a reversal of flow and a “washout” sign.
We were not surprised by the higher incidence of “backflow” in the “octopus” LVA (81% or 13 of 16) relative to the standard LVA because the lymphatic vessels used in the “octopus” technique were qualitatively worse than the ones used in the standard technique, and were mostly of the “contraction type” [19,20]. Without compression, these LVAs would likely not be effective due to their inability to peristalze and generate a favorable lymph-to-vein pressure gradient. Using compression, we were able to convert 100% (13 of 13) of the unfavorable “octopus” LVAs with “backflow” to the favorable, functioning LVA showing “washout”. This finding is encouraging because it suggested that even the damaged “contraction type” lymphatic vessels may be successfully recruited to build functioning LVAs. This allows less restrictive lymphatic vessel recruitment and will lead to increased number of LVAs created per surgery. Furthermore, this means that instead of limiting the LVA procedure only to patients with early diseases [6,21], surgery may be considered even in those with intermediate disease severity who tend to have fewer healthy lymphatic vessels.
In summary, the benefits of immediate compression following the LVA are three-fold:1) it converts nonfunctioning LVAs with retrograde flow to functioning ones with ante grade flow, 2) it augments the flow of the functioning LVAs already demonstrating ante grade flow, and 3) it decreases the restrictive nature of the LVA procedure and allows the surgeon to use moderately disease-affected lymphatic vessels, creating higher numbers of functioning LVAs. Currently, the endpoint of limb compression and the timing of its discontinuation are unknown, and they are being investigated in our ongoing studies.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
Received date: April 30, 2017
Accepted date: October 13, 2017
Published date: May 23, 2018
None
None
© 2018 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Video Video Abstract
Authors report a case of lower extremity lymphedema treated by LVA that preoperatively mapped not only lymphatic vessels by PDE, but also veins and venules using Veinsite™ .
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
ICG lymphography is an invaluable tool in lymphedema management. Both immediate and delayed scans are needed when performing the study. The delayed scan needs to be performed at the time of the lymphographic plateau to appreciate the full extent of the pathology. Using a recumbent cross trainer, the lymphographic plateau can be achieved in 15 minutes following ICG injection. We have found this exercise enhanced ICG lymphography protocol worthwhile of adoption by high volume lymphedema centers to raise diagnostic accuracy and efficiency.
This article holds critical relevance for healthcare professionals, particularly in the fields of microsurgery, oncology, and vascular medicine. It thoroughly examines the diagnostic challenges faced in distinguishing between recurrent lymphedema and deep vein thrombosis in elderly cancer patients following lymphovenous anastomosis surgery. It highlights the significant risk of misdiagnosing deep vein thrombosis as lymphedema, a mistake that can delay critical treatment due to their clinical similarities. The case study of a 79-year-old patient emphasizes the importance of a comprehensive reassessment, considering the patient's entire medical history, including the effects of cancer treatments like immunotherapy. The article stresses the need for a holistic approach to patient management and the utilization of advanced diagnostic tools for accurate diagnosis and treatment. It is essential reading for its insights into the complex dynamics of postoperative care and the critical importance of accurate diagnosis in treating elderly cancer patients effectively.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
Osteoarthritic finger joints are often repaired with joint implants, arthrodesis, or a vascularized interphalangeal joint graft. However, grafts can damage the donor toe. Based on the results of this study, the authors suggest that vascularized distal interphalangeal joint transfers from the second toe may be suitable for reconstructing these defects through microsurgery.
The paper entitled “immediate limb compression following supermicrosurgical lymphaticovenular anastomosis – is it helpful or harmful” written by Chen and colleagues tried to clarify the role of postoperative compression regarding lymphaticovenous anastomosis for lymphedema. They delivered compression immediately after surgery and watch the patency and flow pattern (from the vein to the lymphatics or from the lymphatics to the vein) and found that compression helps to direct the flow into lymphatic- to vein pattern (wash out). Their finding is interesting. The writing of the manuscript is in good shape. The only drawback of the study would be the small sample size and include different methods of anastmosis. However, the interesting finding is still worthy published.
I appreciate the opportunity of reviewing the manuscript of by Chen et al and co-workers, entitled, “Immediate External Limb Compression Following Supermicrosurgical Lymphaticovenular Anastomosis – Is it Helpful or Harmful?” The authors have taken on a very difficult and somewhat controversial subject and clearly have invested a great deal of time and effort in this project. The authors evaluate the immediate effects of applying compression after LVA. In 5 consecutive patients, the authors demonstrate that 94% of anastomoses that initially demonstrated “back flow” were converted to a “washout” (anterograde) pattern. These findings in the OR are presented alongside longer term follow-up. I believe this manuscript is well written and succinct. I believe this manuscript is appropriate and would be of interest to the readership of the International Microsurgery Journal. All the tables and figures are appropriate. While I do believe the manuscript would be strengthened by more patients, this preliminary work is important and I would recommend for publication without revisions.
Chen WF, Bowen M, Ding J. Immediate limb compression following supermicrosurgical lymphaticovenular anastomosis – Is it helpful or harmful? Int Microsurg J 2018;2(1):1. https://doi.org/10.24983/scitemed.imj.2018.00063