Introduction: Supermicrosurgical lymphaticovenular anastomosis (LVA) has become an accepted and effective treatment for lymphedema. Surgeons performing these procedures, however, have been perplexed in determining where to place their incisions to maximize the number of LVAs constructed. Here, we describe our guided approach to incision placement to increase the likelihood of creating a successful LVA at each incision.
Methods: Thirteen consecutive patients underwent LVA for treatment of secondary lymphedema. Incisions were placed using the guided approach in all the patients. Additional incisions were placed using the blind (anatomic) approach when the appropriate number of LVAs had not been achieved. In the guided approach, superficial lymphatics were mapped intraoperatively with indocyanine green (ICG) lymphography and superficial venules were mapped intraoperatively with near-infrared (NIR) vein visualization (Figure 2). Guided incisions were then placed where lymphatic vessels and superficial venules came into close contact or intersected (Figure 3). In the blind approach, incisions were placed along the anatomic course of the cephalic or greater saphenous vein (Figure 3). The number of LVAs constructed using each approach was compared. Fisher’s exact test was used for statistical analysis.
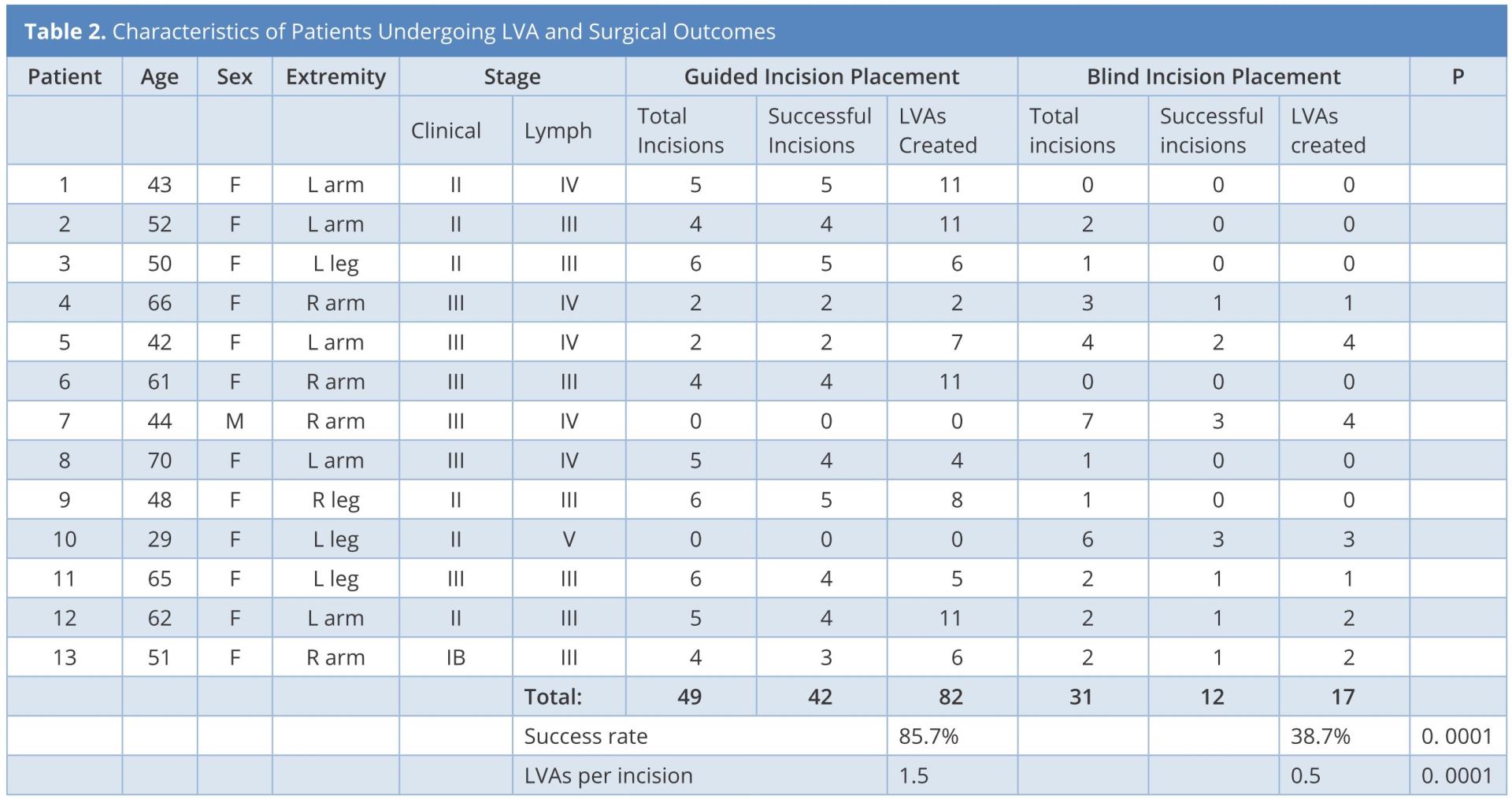
Results: A total of 99 LVAs were created through 80 incisions. Twelve of 31 (39% success) incisions using the blind approach and 42 of 49 (86% success) incisions using the guided approach allowed for successful LVA construction. The guided approach also resulted in more LVAs created per incision (1.7 vs. 0.5, P = 0.0001).
Conclusion: Use of a multimodality image guided approach significantly increases the probability of successful LVA creation at each incision as well as the total number of LVAs that are created within each incision.
Video Abstract
Lymphedema is a chronic and progressive disease that has been historically difficult to manage. Cancer treatments, especially those involving lymph node dissections, have contributed to the increasing prevalence of the disease. Supermicrosurgical lymphaticovenular anastomosis (LVA) has been shown to effectively treat lymphedema [1-4]. In LVA, lymphatic vessels are connected to veins through a series of small skin incisions. The anastomoses create additional outflow conduits to improve lymphatic drainage.
Successful construction of an LVA requires identification of both adequate caliber lymphatic vessels and venules within each incision site. Lymphatic vessels used in LVA must be patent and located near veins of compatible size. If the vein is too large, pressure in the lymphatic vessel will be insufficient to overcome the venous blood flow and the LVA will not function. If the vein is too small, the anastomosis may be technically difficult to complete (currently 0.2 mm is the smallest vessel size used at our institution). One of the difficulties the surgeons encounter while performing LVA is in determining where to place incisions to access veins and lymphatic vessels meeting these criteria.
LVA incisions were historically placed in a blind fashion. Surgeons mapped superficial veins using anatomic landmarks, such as the cephalic vein in the upper extremity and the greater saphenous vein in the lower extremity [4]. To increase the likelihood of encountering lymphatic vessels, LVA incisions were typically clustered distally where tissues were thinner and vessels were present at a higher concentration [1,2]. To improve the success rate of encountering adequate lymphatics and veins at LVA incision sites, surgeons began using intraoperative ICG lymphography guidance [5,6]. Although ICG lymphography allowed the surgeons to encounter lymphatic vessels with higher frequency, it did not improve the probability of finding the nearby venules. This led to combining ICG lymphography and near-infrared (NIR) vein visualization, which had resulted in successful outcomes [7,8]. However, there have not been any studies to our knowledge directly comparing the success rates of this mapped approach and the traditional blind (anatomical) mapping. Here, we describe a method of guided incision placement and compare the rate of successful LVA formation to that found in the blind approach.
Patient Selection and Evaluation
Following IRB approval, the patients at the University of Iowa Hospitals and Clinics who underwent LVA for treatment of secondary lymphedema between July 2015 and December 2015 were recruited for study [9,10]. Disease severity was staged clinically using Campisi criteria (Table 1) and radiographically using indocyanine green (ICG) lymphography staging criteria previously described by Yamamoto et al [11]. Patient assessment was performed preoperatively and at three and six months postoperatively. The assessment included patient-reported relief of symptoms, clinical exam, validated lymphedema quality of life assessment, and ICG lymphography [12].
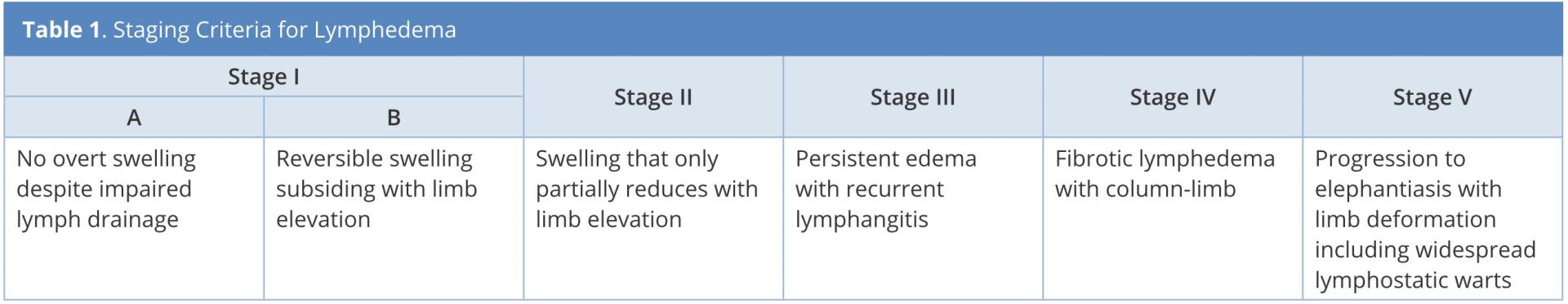
Staging criteria for lymphedema is adapted from Campisi, et al. [5,6].
Vessel Mapping
In the guided approach, superficial lymphatics were mapped intraoperatively with ICG lymphography by injecting 0.1 mL of 0.25% ICG intradermally at the first and second web spaces of the foot or the second and third web spaces of the hand (Figure 1A). The injected limb was scanned with the SPY Elite system (Life-Cell Corp., Bridgewaterer, NJ) immediately following injections to visualize the superficial lymphatic vessels. Lymphatic vessels were marked with a solid line based on injection site (Figure 1B). Additional injections were performed until no drainage from the most recent injection was visualized.
Superficial venules were mapped using the Veinsite (VueTek Scientific®, Gray, ME) near-infrared (NIR) vein visualization and marked with a dotted line (Figure 2C, 3A). Incision sites for LVA were marked where solid and dotted lines intersected or were in close proximity.
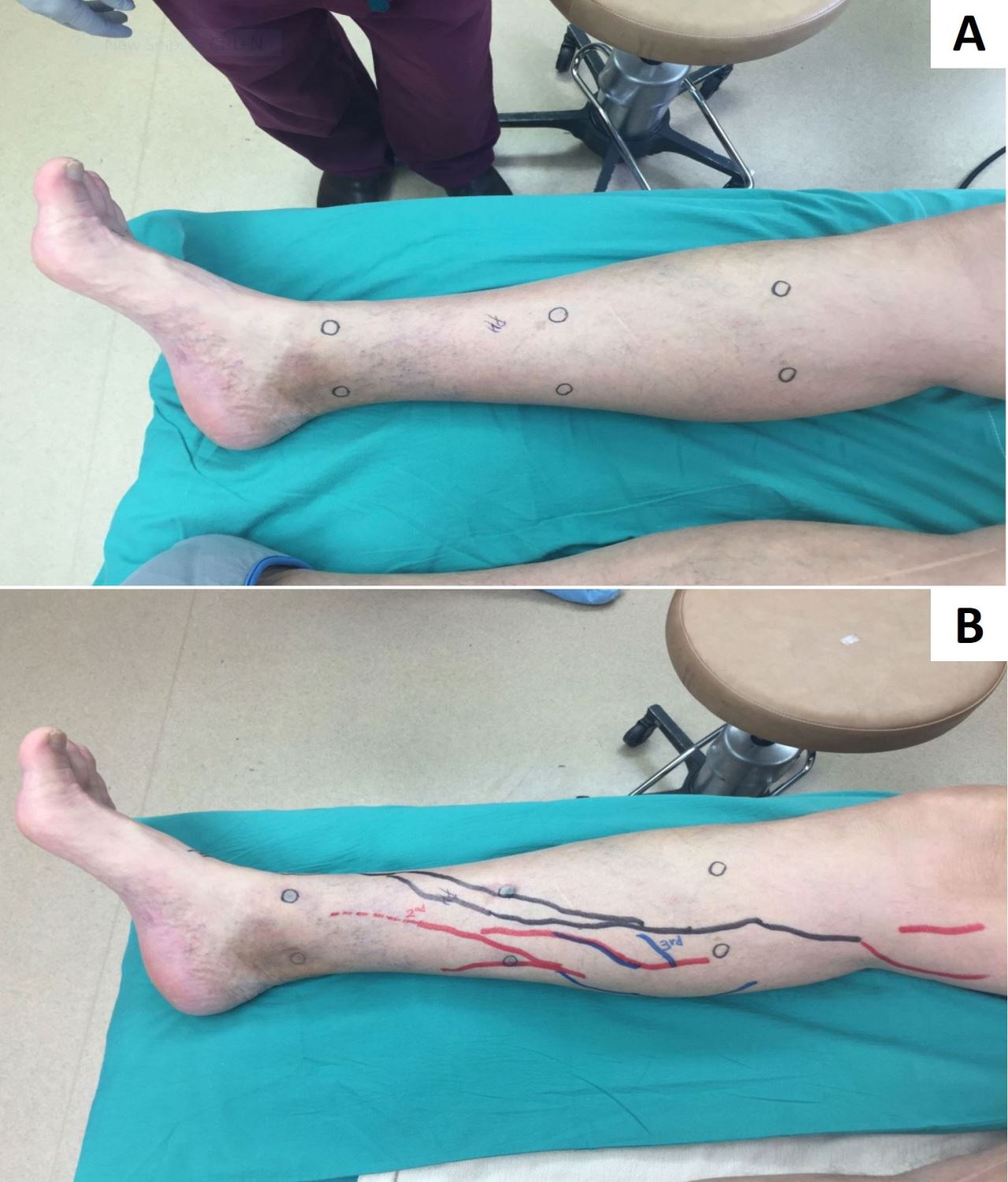
Figure 1. A, marked sites for sequential distal-to-proximal ICG injection. B, lymphatic vessels visualized using ICG lymphography marked with solid lines in colors corresponding to injection site.
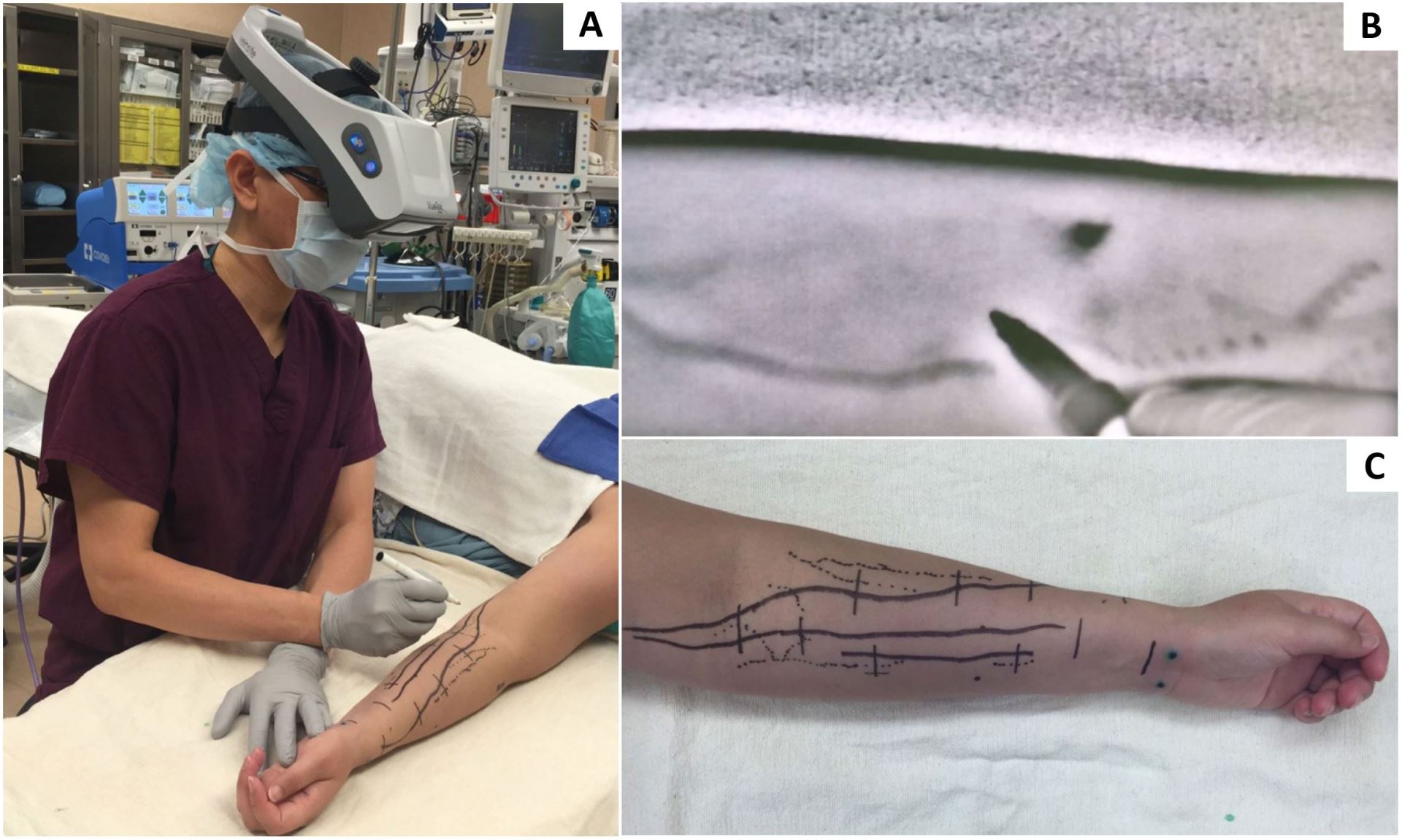
Figure 2. A, marking the superficial veins of upper extremity with the Veinsite (VueTek Scientific®, Gray, ME). B, vein marking with marking pen as viewed through the Veinsite. C, upper extremity with veins (dotted lines) marked.
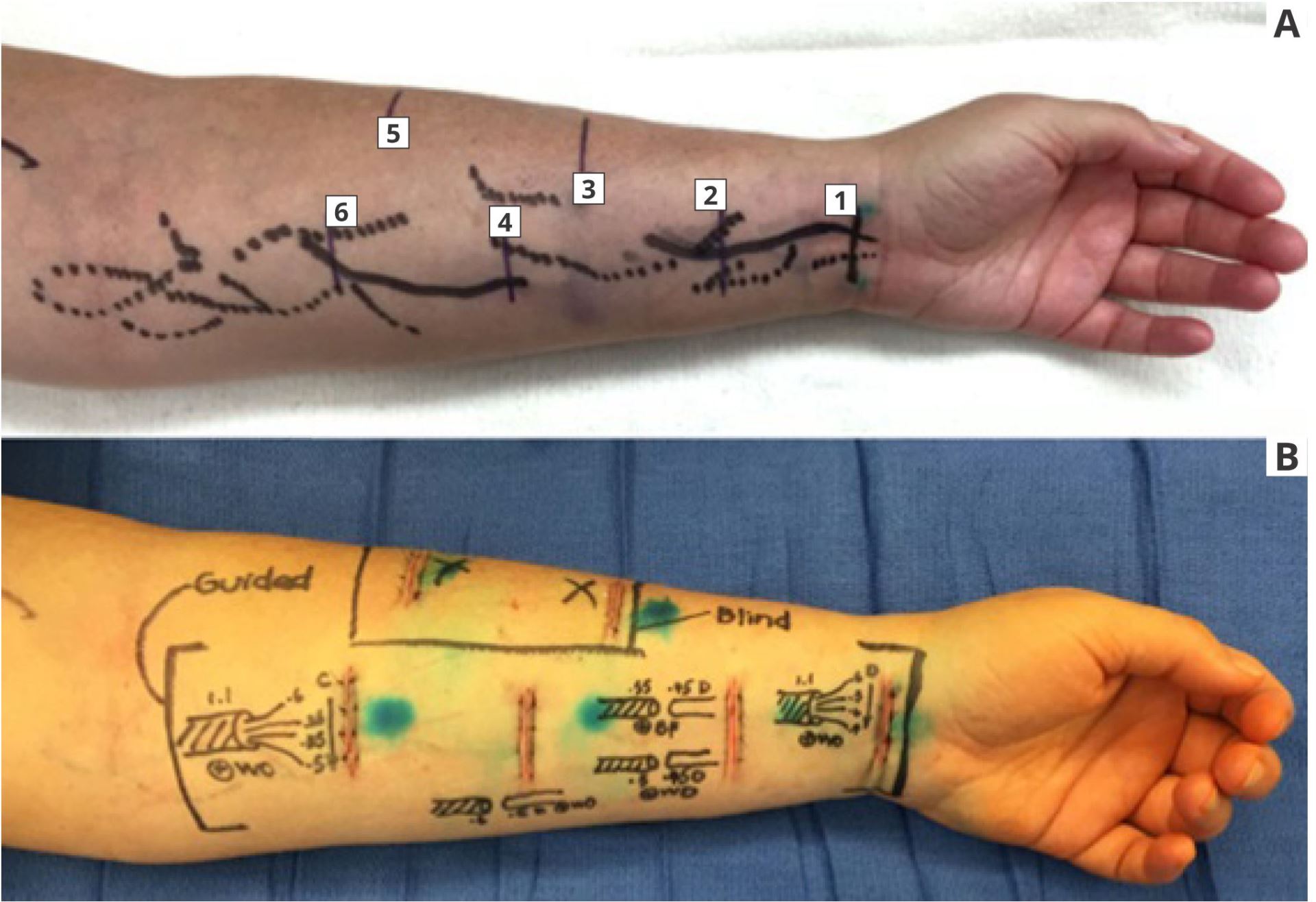
Figure 3. A, guided incisions (1, 2, 4, and 6 on volar forearm) marked at sites of intersecting or nearby veins (dotted lines) and lymphatic vessels
Following incision markings, 0.1 mL of 1% Lymphazurin was injected approximately 2 cm distal to each incision to aid in intraoperative visualization of the lymphatic vessels. Dissection was performed through 3 cm incisions under 18x to 22x magnification with a surgical microscope (Pentero 900; Carl Zeiss, Oberkochen, Germany). Supermicrosurgical instruments (EMI Factory, Kitasakugun, Japan) were used for vessel dissection. Anastomoses were performed using 11-0 and 12-0 Nylon sutures (Crown Jun, 50μ needle, Ichikawashi, Chiba, Japan) using techniques previously described [13-15].
The blind approach to incision placement was utilized when fewer than 10 LVAs were created with the guided approach or when the quality of the lymphatic vessels was insufficient (Table 2). Lymphatic vessel quality was determined visually and categorized as good (normal or ectatic), suboptimal (contracted), or unusable (sclerotic) [16]. When indicated, blind incisions were made following the anatomic course of the cephalic or greater saphenous vein (Figure 3A). Operative times for each case were obtained from the case log and vessel mapping times were recorded. Fisher’s exact test was used for statistical analysis.
Abbreviations: LVA, lymphaticovenous anastomosis; Lymph, lymphangiographic.
Patient Selection and Evaluation
Thirteen patients with lymphedema Campisi stage Ib to III and lymphographic stage II to V met the inclusion criteria (Table 2). All the thirteen patients had uneventful postoperative courses and were discharged one day postoperatively. The follow-up period ranged from five to nine months; no patients were lost to follow up. At their follow-up appointments, all patients reported a decrease in lymphedema-related symptoms that paralleled their improved findings on clinical exam, validated quality of life assessment, and ICG lymphography.
LVA Completion
A total of 99 LVAs were created through 80 incisions by senior author WFC. Forty-two of 49 (86% success) incisions using the guided approach resulted in successful completion of LVA (Table 2). Twelve of 31 (39% success) incisions using the blind approach resulted in successful completion of LVA (Table 2). The guided approach allowed construction of 1.7 LVAs per incision, while the blind approach allowed construction of 0.5 LVAs per incision (Table 2). The average operative time for the thirteen patients was 4.8 ± 0.5 hours and the time spent for mapping vessels was 13 ± 3 minutes.
The goal of surgical treatment of lymphedema with LVA is to provide alternative drainage routes for excessive accumulation of lymph. To successfully construct an LVA, the surgeon must identify both suitable lymphatic vessel (normal, ectactic, or contracted) and suitable vein. Both the lymphatic vessel and vein must be of compatible size and close enough to be connected.
In this patient population, utilization of guided incision placement with multi-modality intraoperative imaging increased successful LVA construction at each incision (86% vs. 39%, P = 0.0001) and also increased the number of LVAs constructed within each incision (1.7 vs. 0.5, P = 0.0001). Although mapping with ICG lymphography and NIR added 10-15 minutes to the operative time, the overall operative time decreased when this technique was used. We attributed this to the ability to complete more successful LVAs using fewer incisions. While lymphatic and venous mapping equipment must be purchased to perform the guided incision approach, this is a one-time fixed cost. Thereafter, performing venous mapping has no additional expense. Performing ICG lymphography requires ICG dye and an injection syringe with needle, neither of which adds appreciable cost to the LVA procedure.
We acknowledge that by using the blind approach only when the lymphatic vessels were inadequate in quality or LVAs were inadequate in number, a degree of selection bias was present in our study design. The study design was selected because by using the guided incision placement in every patient when able, we could create more LVAs and optimize the individual patient outcomes following surgery.
While it has been demonstrated that the patient outcomes are improved when more LVAs are made [17,18], the optimal number of LVAs is unknown. The goal is to maximize lymphatic drainage pathways. We recommend surgeons who are skilled in various anastomotic configurations. Too often, surgeons rely on end-to-end anastomoses, which create fewer drainage pathways than other configurations. Currently at our institution, we determine surgical endpoint based on quality and quantity of LVAs constructed, patient anesthesia time, and surgeon fatigue. The average operative time of the patients in this study (4.8 hours ± 0.5 hours) was based on this approach. In future studies, we hope to explore criteria for a more definitive LVA operation endpoint.
Many surgeons who perform the LVA consider the absence of linear pattern finding on ICG lymphography to be a contraindication to the procedure. This study demonstrated that even in the absence of linear pattern ICG lymphographic findings, we still identified suitable lymphatic vessels and created successful LVAs, albeit at a lower success rate (Table 2). Based on these findings, we do not consider the presence of a linear ICG lymphographic pattern to be a prerequisite for successful surgery. We prefer a guided approach for LVA when possible but still offer the procedure in patients without linear patterns on ICG lymphography.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
Received date: May 12, 2017
Accepted date: October 18, 2017
Published date: January 02, 2018
None
None
© 2018 The Author(s). This is an open-access article distribut- ed under the terms of the Creative Commons Attribution 4.0 Internation- al License (CC-BY).
Video Abstract
Authors report a case of lower extremity lymphedema treated by LVA that preoperatively mapped not only lymphatic vessels by PDE, but also veins and venules using Veinsite™ .
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
ICG lymphography is an invaluable tool in lymphedema management. Both immediate and delayed scans are needed when performing the study. The delayed scan needs to be performed at the time of the lymphographic plateau to appreciate the full extent of the pathology. Using a recumbent cross trainer, the lymphographic plateau can be achieved in 15 minutes following ICG injection. We have found this exercise enhanced ICG lymphography protocol worthwhile of adoption by high volume lymphedema centers to raise diagnostic accuracy and efficiency.
This article holds critical relevance for healthcare professionals, particularly in the fields of microsurgery, oncology, and vascular medicine. It thoroughly examines the diagnostic challenges faced in distinguishing between recurrent lymphedema and deep vein thrombosis in elderly cancer patients following lymphovenous anastomosis surgery. It highlights the significant risk of misdiagnosing deep vein thrombosis as lymphedema, a mistake that can delay critical treatment due to their clinical similarities. The case study of a 79-year-old patient emphasizes the importance of a comprehensive reassessment, considering the patient's entire medical history, including the effects of cancer treatments like immunotherapy. The article stresses the need for a holistic approach to patient management and the utilization of advanced diagnostic tools for accurate diagnosis and treatment. It is essential reading for its insights into the complex dynamics of postoperative care and the critical importance of accurate diagnosis in treating elderly cancer patients effectively.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
Osteoarthritic finger joints are often repaired with joint implants, arthrodesis, or a vascularized interphalangeal joint graft. However, grafts can damage the donor toe. Based on the results of this study, the authors suggest that vascularized distal interphalangeal joint transfers from the second toe may be suitable for reconstructing these defects through microsurgery.
IRB: approval obtained.
Sampling: consecutive patients meeting the inclusion criteria.
Study design: well defined diagnostic criteria and follow up protocol.
Data analysis: appropriate statistical test, adequate number of tables and figures.
The paper selected an important issue which is so helpful for planning surgery in a very safe way, and is really presented in a very precise way by the authors. The way of assessment of the cases and the methods of evaluating the results were fantastic. I would highly recommend accepting it for publication.
The authors describe superiority of a multimodality approach over a blind approach. While their conclusions seem obvious, the multimodality approach itself is recommended whenever possible. However, there are several points that the authors should make clear.
Accepted for publication
Authors have made the necessary changes. Publish the article.
Hawkes PJ, McNurlen M, Bowen M, Chen WF. Strategic incision placement to facilitate successful supermicrosurgical lymphaticovenular anastomoses. Int Microsurg J 2018;1(3):5. https://doi.org/10.24983/scitemed.imj.2018.00049