Background: Multidisciplinary approach is essential for reconstructing patients with chest wall tumor, especially in palliative settings. There are few studies that describe the essential role of a plastic surgeon for this entity. The main challenges are not only the aesthetic appearance but also the functional outcomes attained by various tissue transfer techniques. This study presents reconstructive options as one of the mainstay therapies of patients with chest wall tumors.
Methods: Fifteen patients, aged 13 to 65 years, who underwent chest wall reconstruction at Dharmais National Cancer Center, Jakarta, Indonesia from 2014 to 2018 were reviewed. The outcomes such as complications, recurrence, and mortality were recorded. The follow-up period ranged from 6 to 24 months.
Results: Pedicled and free flaps were the preferred treatment options to cover the chest wall defect following wide resection. Flap modalities such as latissimus dorsi (LD), anterolateral thigh, transverse rectus abdominal muscle, and external oblique muscle were applied. Skin grafts were frequently combined in LD flap to cover the muscle flap. In large composite defects in the precordial area with sternum/ribs resection, titanium mesh was used.
Conclusion: This series has shown that the pedicled and free flaps are reliable modalities for reconstruction following chest wall tumor ablation, with improvement in aesthetics and functionality. Immediate reconstructive surgery post-cancer ablation has been proven to be safe and reliable without increasing the number of morbidities, even in palliative settings.
Oncologic processes of the chest wall mainly comprise of primary thoracic wall tumor and metastatic lesion from adjacent tissues and organs. The most common primary tumors originate from bones and soft-tissue sarcomas, which account for 55% and 45% respectively [1,2]. On the other hand, the metastatic lesions commonly occur from adjacent tissues malignancies, such as breast cancer and lung cancer. It has been suggested that the treatment approach may include surgical resection of the defects, which is usually followed by chemotherapy and/or radiation depending on the histological entity. Nowadays, wide surgical excision towards chest wall tumors has resulted in the increased long-term survival of the patients [3,4].
Studies have shown that reconstruction of chest wall defects is required to reduce morbidity and mortality following tumor excision [3,4]. In the palliative setting, resection of painful, odor-intensive, and bleeding tumors with subsequent chest wall reconstruction seems to be an option to increase the quality of life in a period of time. Nevertheless, three main issues should be considered in order to obtain optimal surgical treatment for chest wall tumor, namely, the extension of resection, restoration of skeletal stability, and soft tissue coverage [5,6]. These issues require a multidisciplinary approach, which involves cooperation between oncologic, thoracic, and plastic surgeons. The primary goal in this multidisciplinary approach is to earn the desired results in terms of oncological, functional, and aesthetic fields. In the surgical field, plastic surgery procedures improve the patient’s condition by refining the local tissue situation [7].
For chest wall defects, various soft tissue transfer techniques are required to cover the post-excision defects. Most often, muscle or musculocutaneous flaps are used in chest wall reconstruction. More advanced techniques such as free tissue transfer (free flap) and multiple simultaneous flaps are needed to reconstruct larger defects.
The purpose of this study is to review the reconstruction modalities within the multidisciplinary approach for chest wall reconstruction. We have also proposed an algorithm that is formulated based on our experience to manage chest wall defects following cancer resection.
Fifteen patients, aged 13 to 65 years, who underwent chest wall reconstruction at Dharmais National Cancer Center, Jakarta, Indonesia from 2014 to 2018 were reviewed. Patients were observed carefully for 5 days and given antibiotics and analgesia following the surgery. Monitoring to determine flaps vitality was based on the color of the skin/skin graft, skin turgor, temperature, and capillary refill time.
Complications, recurrence, period of follow up, and mortality data based on diagnosis were recorded. The follow-up period ranged from 6 to 24 months. This was a retrospective study and all patients had given their informed consent prior to the study.
Records of fifteen patients who underwent chest wall reconstruction at our institution from 2014 to 2018 were reviewed. Patients’ demographic data (gender, age, pathologic diagnosis, oncologic status, and reconstructive techniques) are reported in Table 1.
There were 11 females and 4 males. The age ranged from 13 to 61 years with an average age of 47.2 ± (13.59) years. All patients underwent surgery for soft-tissue cancers: 11 patients had breast cancer, 1 had thyroid carcinoma, 1 had chondroma, 1 had primitive neuroectodermal tumor (PNET), and 1 patient had skin carcinoma. Some of the defects included lung lobule and/or bony component such as clavicle, sternum and/or ribs. The patients were divided into 5 groups according to the reconstructive techniques and variables such as the size of the chest wall defect and the number of ribs and/or other bony materials resected. The pathological conditions of 9 patients resulted from the recurrence of the disease. Majority of the patients received neo-adjuvant chemotherapy preoperatively.
The majority of the patients were reconstructed in a single-stage fashion; only 1 patient had to undergo two consecutive reconstruction procedures due to cancer recurrence. Definitive chest wall reconstruction was achieved with a total of 18 flaps in 15 patients (Table 1). Varieties of flaps were used, which included fasciocutaneous, musculocutaneous, and muscle flaps. The only free flap used in this study was in the form of anterior lateral thigh (ALT) flap, which was used in 2 cases. Anastomoses of blood vessels in free tissue transfer procedure were achieved using microsurgery techniques. Titanium mesh was incorporated in the reconstruction of the chest wall specifically when bony materials in the precordial area were resected. Surgery was always performed under general anesthesia with endotracheal intubation. Drainage of the surgical site was obtained using simple fenestrated drain. Drains were removed after the residue reduced to less than 30 ml.
As shown in Table 2, the patients were organized into 5 groups based on the applied flaps: 1) anterior lateral thigh (free flap), 2) external oblique, 3) latissimus dorsi, 4) transverse rectus abdominis, and 5) multiple. In Figure 3 to 8, five types of applied flaps in this study were presented. One patient who experienced two-stage operation was included in each accomplished-reconstruction-procedure group on the patient (latissimus dorsi and external oblique group).
Latissimus dorsi (LD) flap coverage was the smallest among other types of flaps recorded, which had covered about 121.4 cm2 defect area. However, it was the most common muscle or musculocutaneous accomplished in this study, involving 7 patients. The largest defect area, which was 300 cm2 on average, was covered with multiple flaps consisting of LD and external oblique muscle flaps in two patients. ALT free flap was the choice to cover the average of 276.5 cm2 defect area. Higher numbers of resected costae were also associated with ALT free flap, which comprised of 4 ribs in each patient. In this group, additionally, a forequarter amputation involving scapula and shoulder girdle in one patient and upper lung lobe resection in another patient were done due to the metastatic progress. The third largest defect group was closed with pedicled transverse rectus abdominis myocutaneous (TRAM) flap, which accounted for 184 cm2 defect area. External oblique flap, on the other hand, was used to cover the defect area with the average size of about 136.5 cm2 , which was almost the same size of the defect with LD flap covering.
In the LD flap group, split-thickness skin graft was used in 2 out of 7 cases to cover the exposed muscle flaps. The rest of the cases were applied with LD flap with skin paddle or closed primarily. There was no remarkable complication recorded in our cases (Table 3). The surgical area was evaluated based on the evidence of flap loss, infection, and dehiscence. However, none of the issues occurred in these cases.
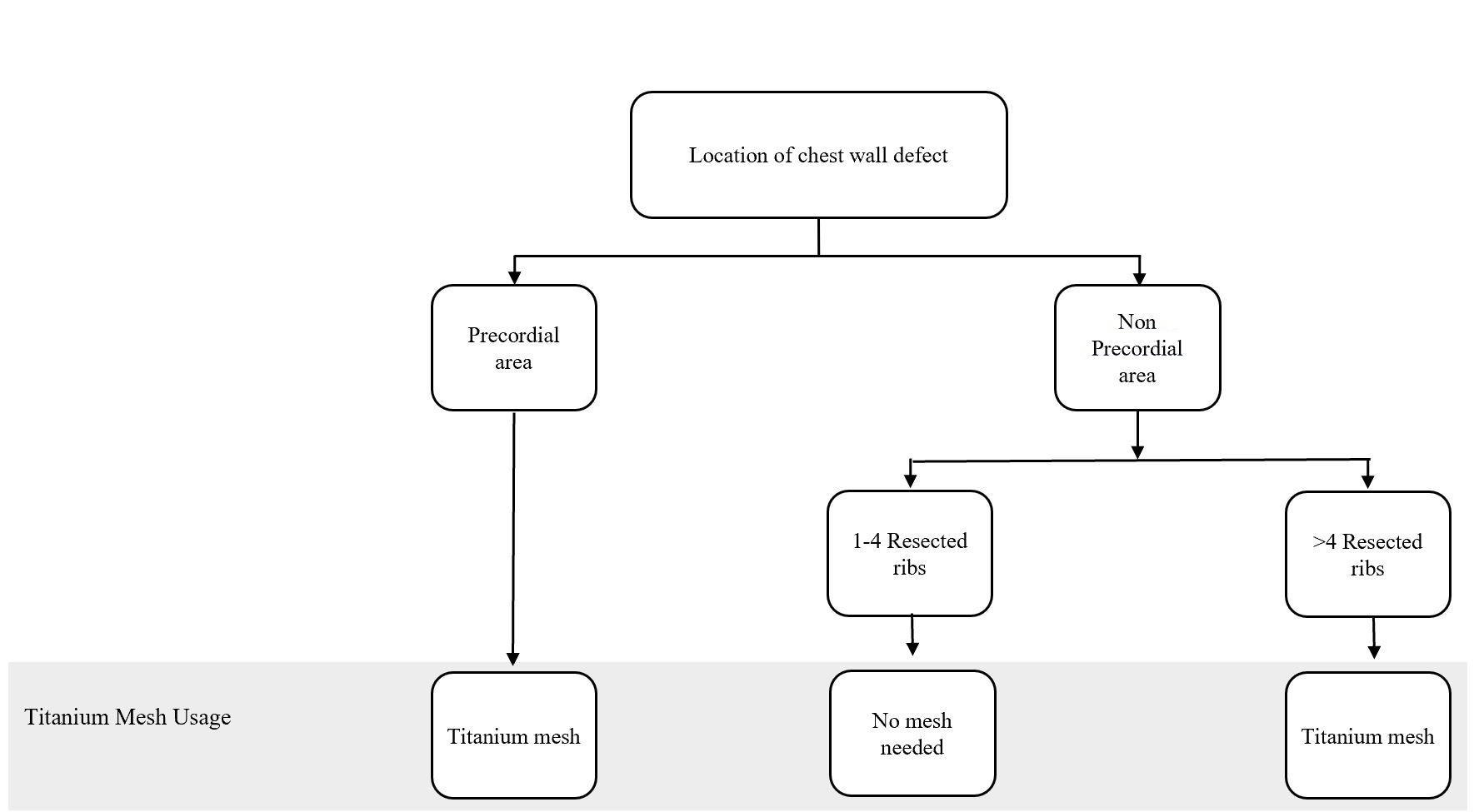
Bony wall reconstruction using titanium mesh was achieved when the pericardial area was involved or the resected ribs reached greater than 4 ribs in a single procedure. Major complications involved the disturbance in chest function, such as flail chest and paradoxical breathing, which covered ventilation problem. None of the complications was also shown in the patients. Nevertheless, 4 patients had a medical complication in the form of their oncological progressivity
After 24 months of follow up, 12 patients remained alive. Recurrence of the disease occurred in 4 cases and currently only one patient diagnosed with PNET was alive. Two patients with breast cancer and one patient with skin cancer died during the 6 months follow-up due to the progressivity of cancer (Table 4).
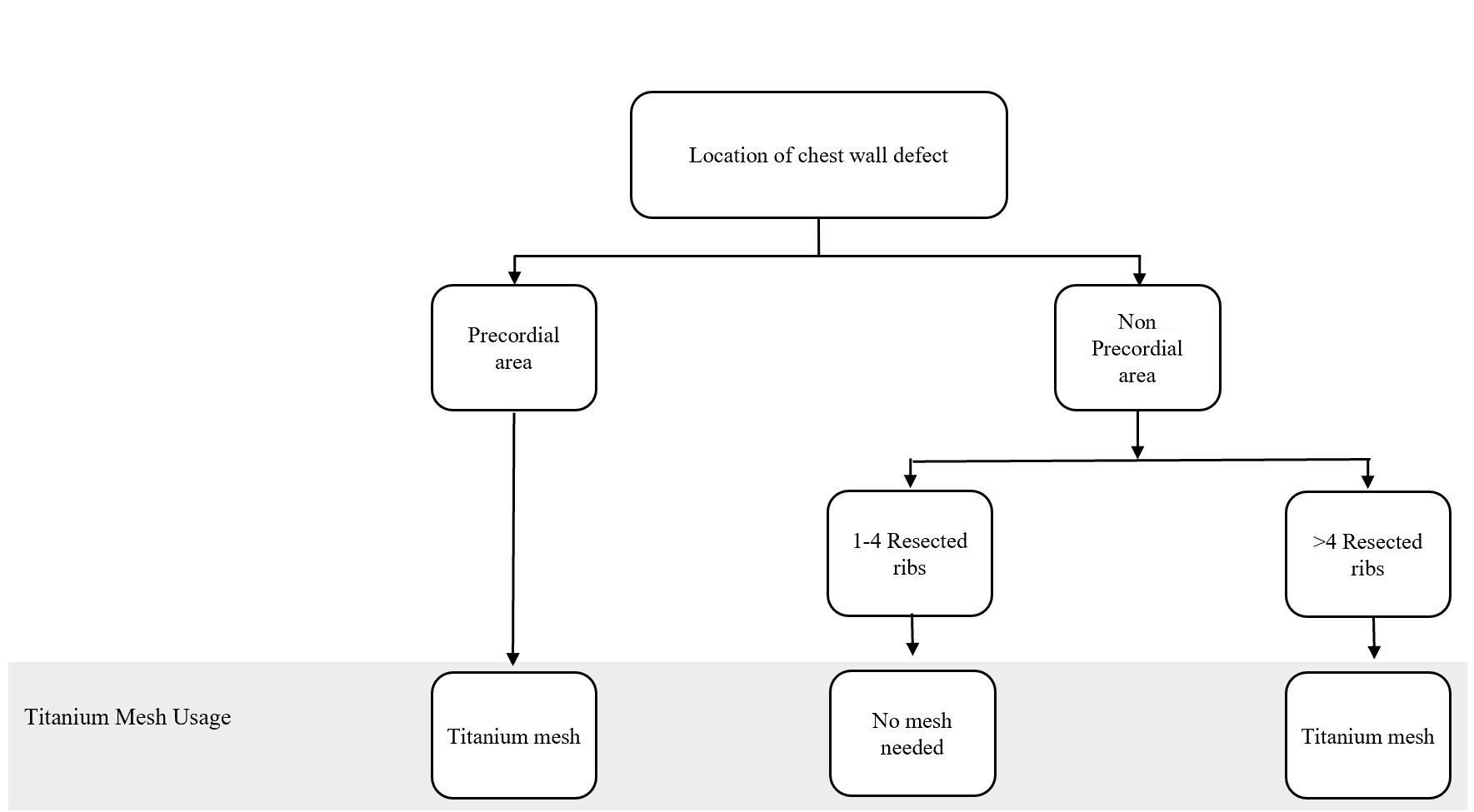
Figure 1. Algorithm for titanium mesh utilization in chest wall reconstruction procedure. (Adapted from Chang et al. [10] with minor differences based on our experiences).
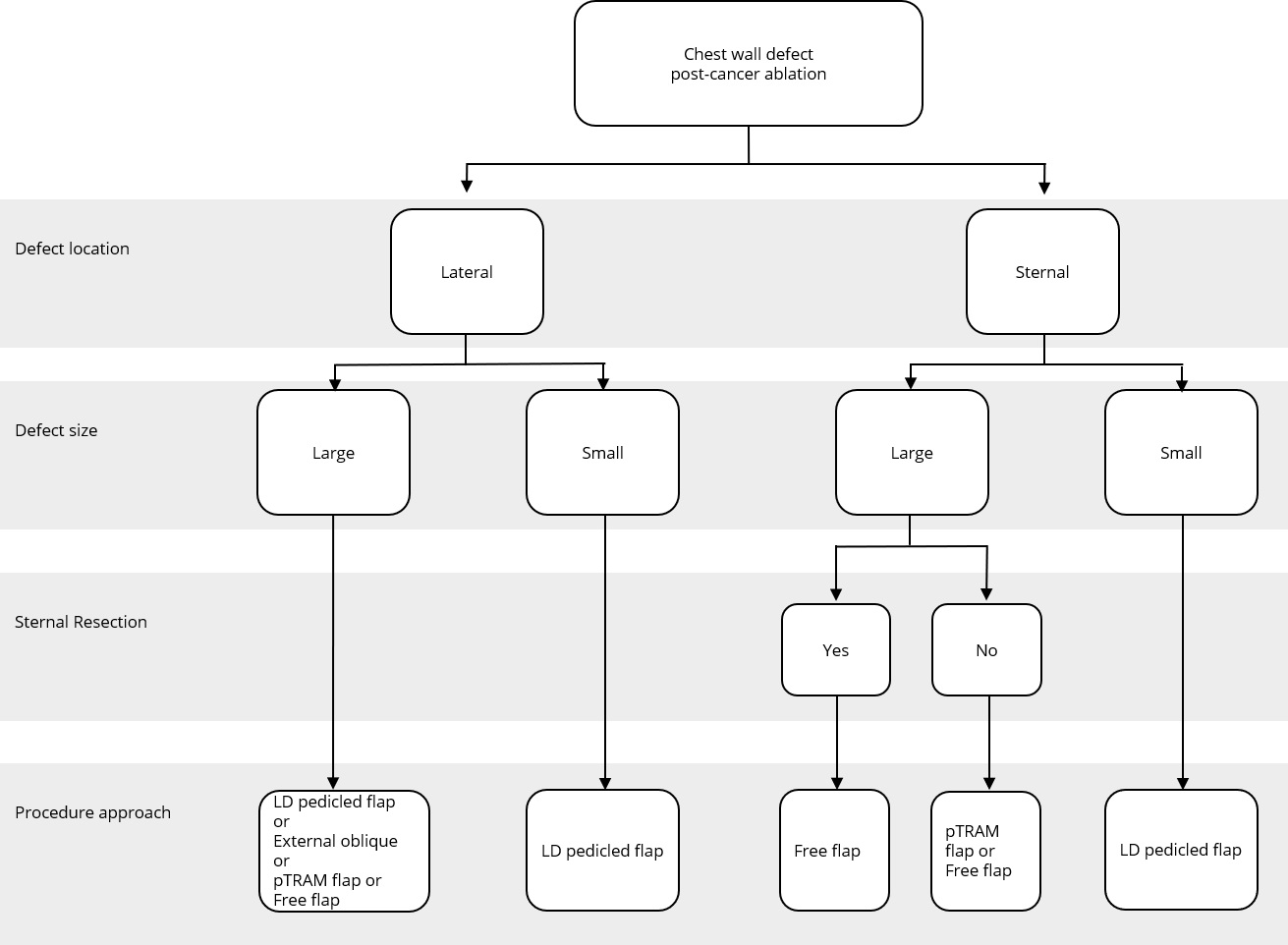
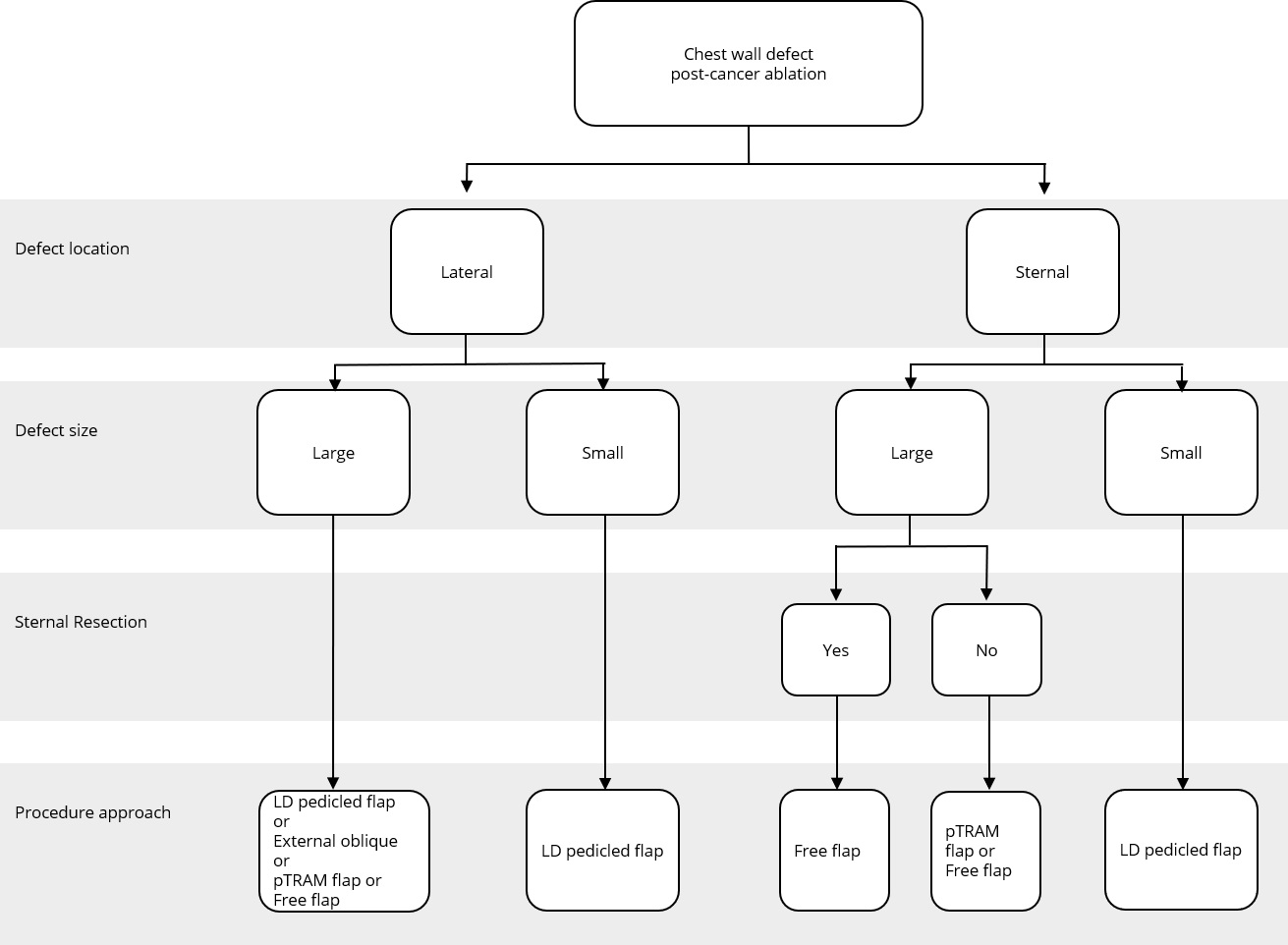
Figure 2. Reconstructive algorithm for chest wall defect following cancer ablation. (Adapted from Chang et al. [10] with minor differences based on our experiences). LD, latissimus dorsi; pTRAM, pedicled transverse rectus abdominis myocutaneous.
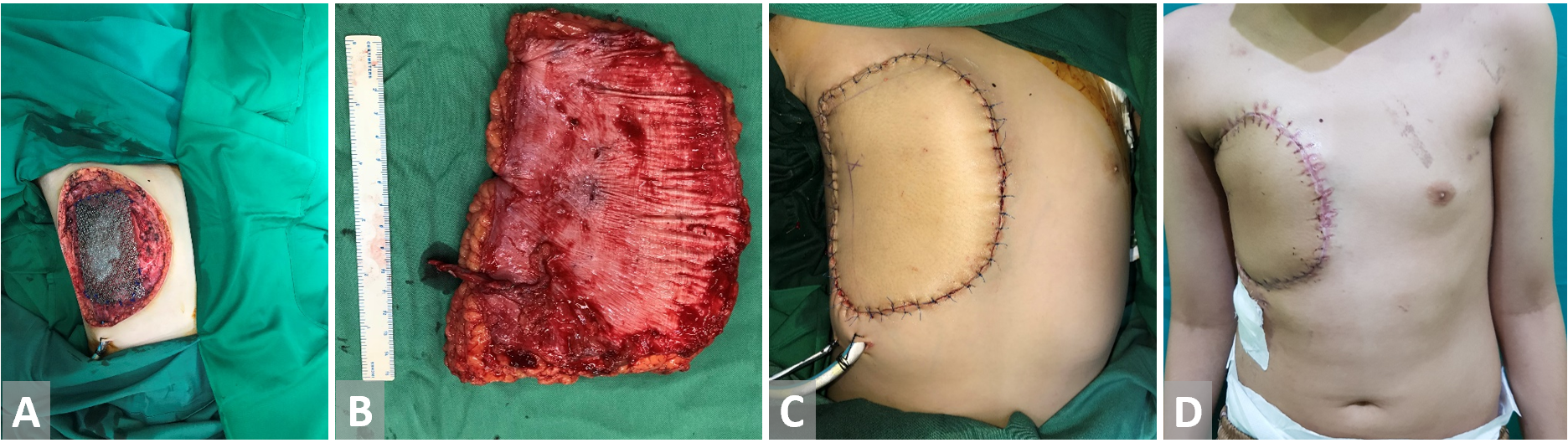
Figure 3. (A) Anterior chest wall resection in a patient with primitive neuroectodermal tumor. Bone defect is covered with titanium mesh. (B) Anterolateral thigh free flap is harvested. (C, D) Reconstruction with anterolateral thigh free flap.
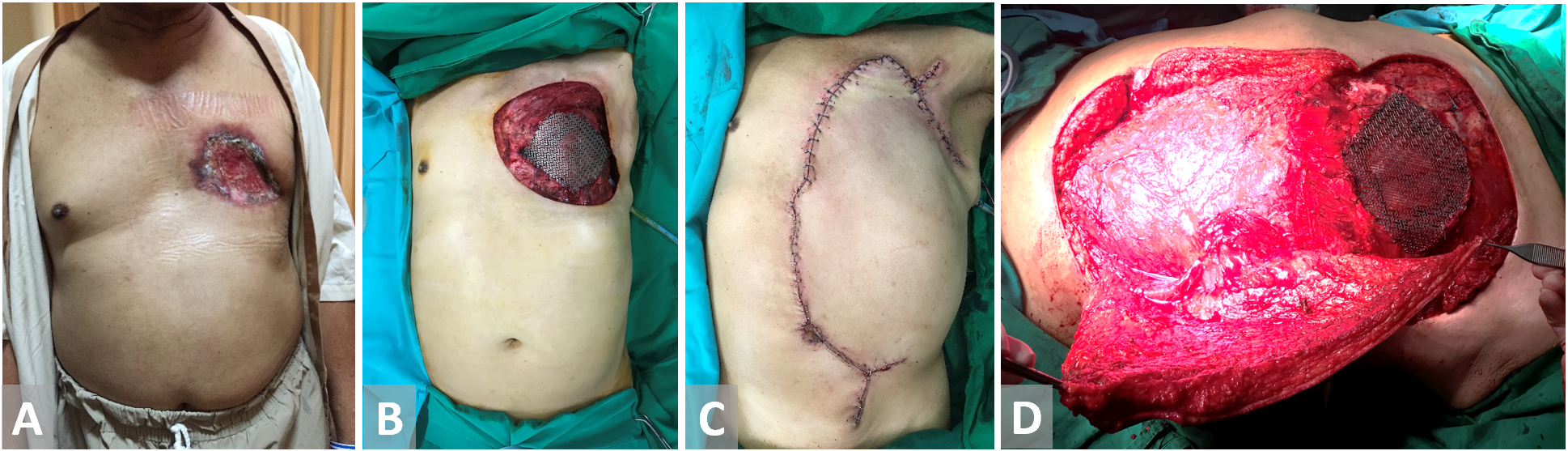
Figure 4. (A, B) Chest wall defect in a male patient with breast cancer. Bony material defect in precordial is protected with titanium mesh. (C, D) External oblique flap is harvested and fully inset.
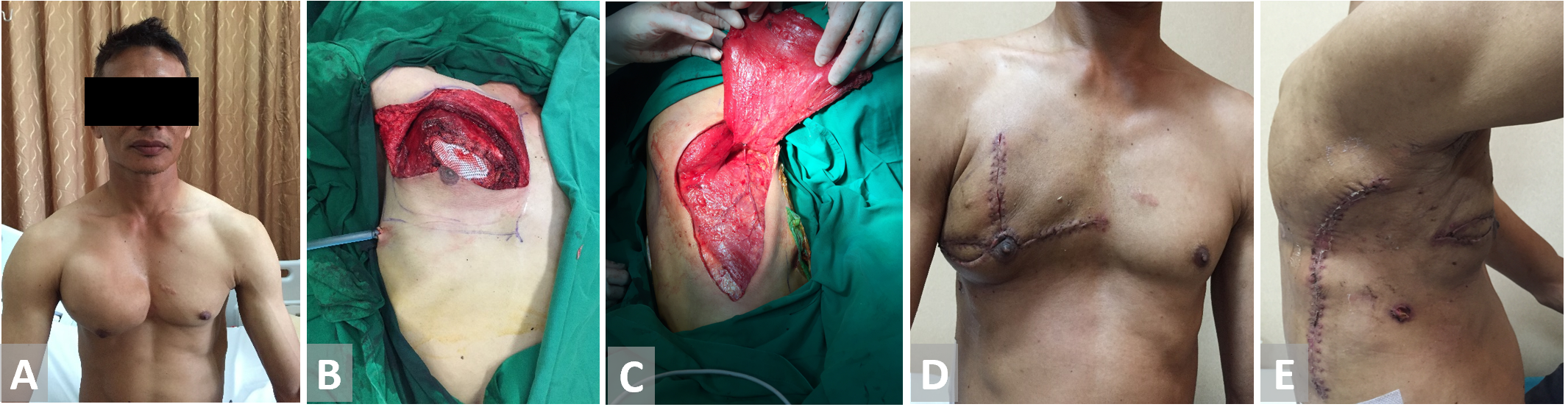
Figure 5. (A, B) Resection of the chest wall in a patient with thorax chondroma. (C) Latissimus dorsi flap is harvested and fully inset. (D, E) Latissimus dorsi flap on 3 weeks follow-up.
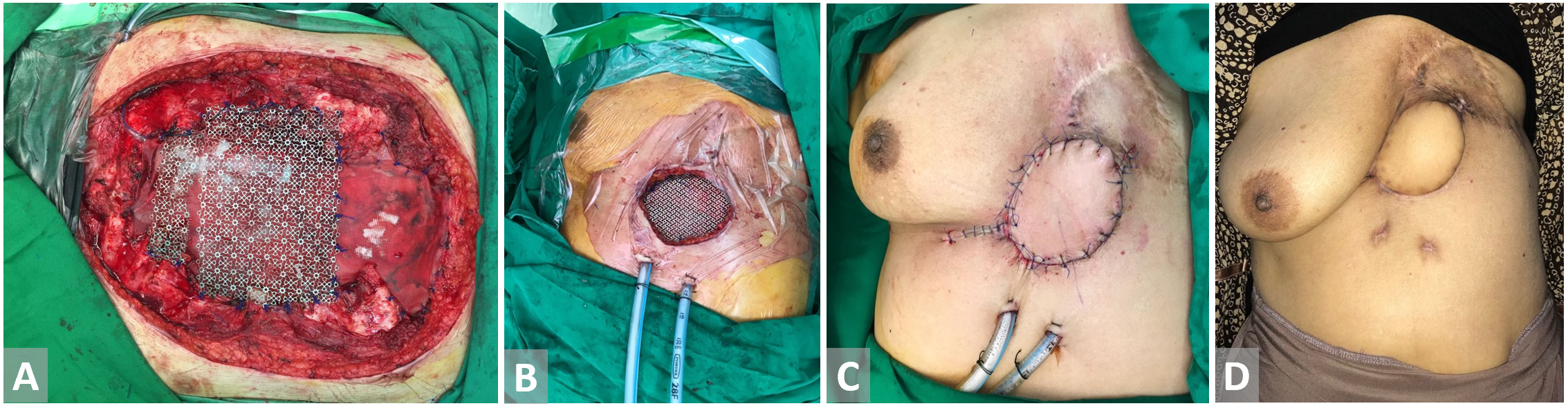
Figure 6. (A, B) Defect in the precordial area post breast cancer excision. Titanium mesh is inset. (C) Latissimus dorsi flap with skin paddle is harvested and fully inset. (D) Latissimus dorsi flap with skin paddle on 3 weeks follow-up.
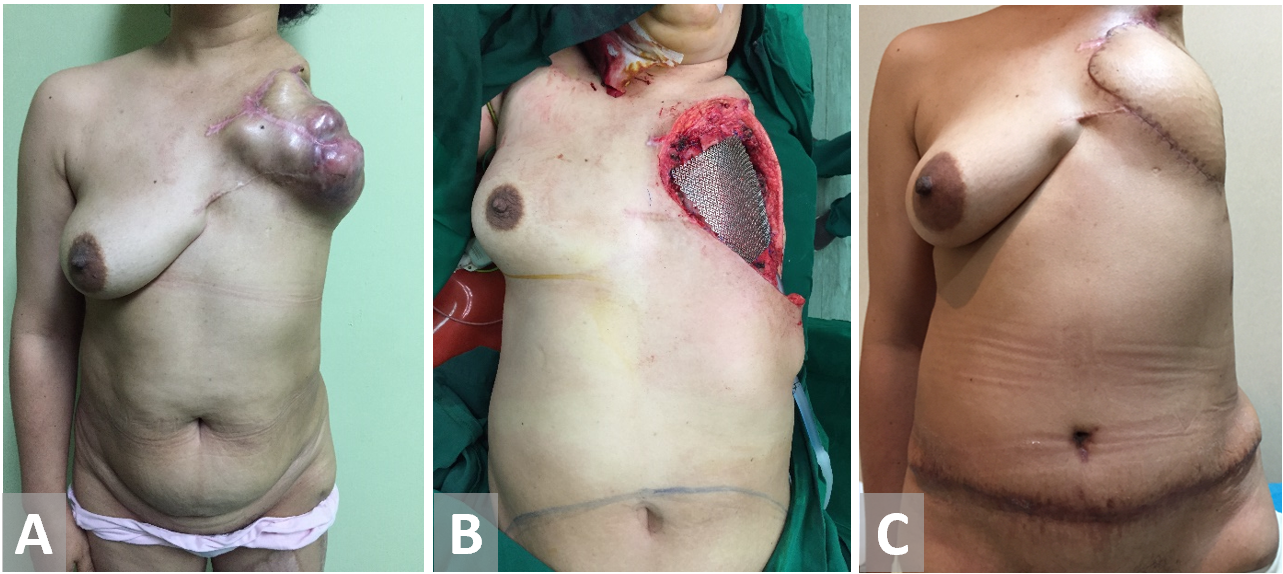
Figure 7. (A, B) Chest wall resection in a female patient with recurrent breast cancer. Forequarter amputation has been terminated on previous surgery. (C) Transverse rectus abdominis muscle reconstruction.
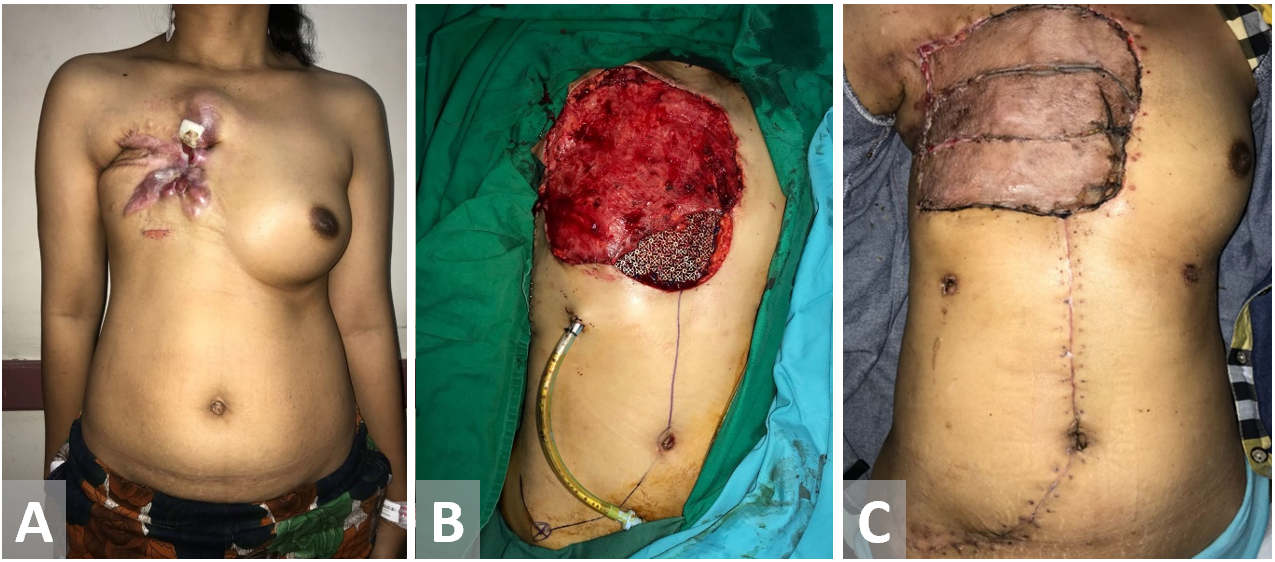
Figure 8. Female patient with recurrent breast cancer. Chest wall is resected. Latissimus dorsi and external oblique muscle flap have been used as the defect coverage. Exposed Latissimus dorsi muscle flap is closed with a split-thickness sk in graft.
This study demonstrates the full spectrum of the complexity of chest wall defects for reconstruction from a tertiary cancer center in Indonesia. The benefit of this single institutional review derives in part from the active role that the surgical treatment plays in the care of oncologic disease of the chest wall. Aggressive extirpation of the tumors results in extensive defects requiring definitive coverage. Such defects most likely qualify for inclusion in the level of highest complexity for reconstruction.
Demographic Data
The most common indication for chest wall reconstruction in our study was breast cancer (73 percent). This demographic data is equivalent to the latest trends showing breast cancer has the highest incidence in Indonesia [8] and the second most common site of cancer in the world [9].
The most flaps used in this series were Latissimus dorsi flaps. Our result was in agreement with a study conducted by Chang et al. [10]. They evaluated 113 patients throughout ten years where the most frequent diagnoses were breast cancer, followed by sarcoma. The type of reconstruction depended on the size and location of the defect, the rotation range of possible local flaps or the availability of vessels for anastomoses. The most commonly used flaps were latissimus dorsi, rectus abdominis, pectoralis major, and external oblique. Microsurgical flaps were used in 11% of their cases [10,11].
Titanium Mesh Usage
In particular, the following defect locations are at the risk of herniation or paradoxical breathing: total or subtotal sternal resection including several ribs bilaterally, high lateral resection (more than 3-4 ribs), or forequarter amputation with resection of 3 or more ribs. In these cases, respiratory problems might arise if the stability is not adequate at the earliest, following the postoperative period [12]. Krall et al. reported that forty patients undergoing a chest wall reconstruction with mesh and flap recovered better significantly with a shorter time on respiratory support and shorter hospital stay compared to those having only flaps reconstruction [13]. Chang et al. proposed an algorithm regarding bony reconstruction on the chest wall: when less than 4 ribs were resected, a mesh-only reconstruction should be applied, whereas when 4 or more ribs and/or a sternal resection were performed, mesh with methyl methacrylate should be used to reconstruct the defect [10]. Nevertheless, we have a limitation in following the guidelines from the previous studies. One of our challenges is regarding national health insurance. The budget has been tightly regulated, including the use of mesh for bony reconstruction.
The algorithm showed in Figure 1 was adapted from Chang et al. [10] and further adjusted based on our experience. Based on this algorithm, we constructed the bony wall only if the pericardial area was exposed, to solely protect the cardiac; and, if the resected costae reached greater than four resected ribs, this might cause some respiratory problems. However, the study conducted by Makboul et al. reported that the use of alloplastic material for structuring was not necessary; they were using only latissimus dorsi flaps to close the defects [11]. This result was in concordance with our cases, where none of the patients showed any breathing complications involving disturbance in chest function, such as flail chest and paradoxical breathing, which covered ventilation problem. However, we only evaluated based on the patients’ clinical symptoms and physical examination within the follow-up period. Further studies, which focus more on the skeletal reconstruction and following ventilation function such as evaluation of lung function test, are paramount to elaborate the work.
Algorithm of Flap
A simple flap choice algorithm after chest wall tumor ablation has been proposed in Figure 2. We adapted the current flap guideline by Chang et al. [10] with some small changes based on our experience. Assessment of the defect location was done in the first place, namely, lateral or sternal area. Size of the defect was determined further. Large defect comprised of defect area ≥300 cm2 while small defect had less than 300 cm2 area. In cases where the defects were located in the middle chest area (sternal), evidence of sternal resection should be recorded. There is a strong relation between resected sternal with the availability of artery internal thoracic. If sternal resection (with the removal of adjacent ribs) was performed, artery internal thoracic was removed along with the resected bone; hence, the choice of the flap would be more restricted. Pedicled TRAM flap or deep inferior epigastric artery perforator flap could not be used in this condition since the blood supply was coming through artery deep inferior epigastric as the continuation of artery internal thoracic.
The anatomic location of the defect in a central, superior, or lateral position most likely determines the choice of the flap. For example, in our series, most cases in which the sternum was resected were reconstructed with latissimus dorsi or rectus abdominis flaps, which could be easily transposed into the centrally localized defect.
Our study concludes the safety and reliability of flap reconstruction of chest wall defects. In each of the cases included in our series, coverage of open wounds of the chest wall was done in a single stage. Extensive defects resulting from the extirpation of oncologic disease were reconstructed in the same operative setting without delay. There was no hesitation to use all advanced techniques at our disposal to attain complete coverage. Microsurgical free tissue transfer was used to provide well-vascularized coverage with distant donor tissues. There was no total flap loss.
Palliative Surgery Setting
Several cases of our series were already in terminal stage involving the palliative team to provide their needs at the end of life stage. Our institution, however, also has a palliative surgery approach. We believe every tumor which limits the patients' activities need to be resected and the defect following the surgery should have proper coverage. However, this procedure should be done after giving informed consent to the patient. Our main aim in these typical cases is to increase the quality of life of the patients.
Reconstruction of chest wall defects by plastic surgeon continues to pose interesting challenges. This, however, requires the multidisciplinary works of other specialties such as oncologic surgeon and thoracic surgeon. Oncologic surgeon together with the thoracic surgeon will excise the tumor, providing the free-tumor-margin, while maintaining the physiological function of the chest wall. This work is continued by closing the defect using local or free flap by the plastic surgeon. These multimodal therapies of chest wall tumors in our institution has been shown to improved local disease control and minimal morbidity, which contributes to full recovery after oncologic surgery. Such coverage could be safely and successfully accomplished in a single stage. This study was conducted in a tertiary center in Indonesia; hence, it represented the current management to reconstruct chest wall tumor in Indonesia as a developing country. Nevertheless, the national health insurance only covered several procedures which were thought to be important; hence, additional test in the follow-up period cannot be obtained. The test included the spirometry test, which was essential to evaluate the respiratory function. Further studies are needed to elaborate more cases and several intended tests in the follow-up period to strengthen the work.
Received date: January 21, 2019
Accepted date: March 26, 2019
Published date: August 12, 2019
Devianti and Mukarramah contributed equally to this work.
The authors acknowledge Dr. Mohamad Rachadian Ramadan’s expert assistance in the manuscript preparation.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2019 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Radiation forms a vital part of neoadjuvant treatment in locally advanced rectal cancer (LARC) and recurrent rectal cancers. The adverse effects of radiation are well recognized; however, radiation-induced perforation at the tumour site is very rare and is poorly understood. A symptomatic rectal perforation requires an emergency surgical intervention. However, it may present silently and can give rise to suspicion of disease progression and/or residual disease on imaging. Authors present two cases of silent perforations. Both gave rise to a considerable diagnostic dilemma, which was resolved by careful evaluation with MRI.
The high incidence of brain metastasis and the comparison of metastatic and non-metastatic phenotypes indicate an active crosstalk of brain metastatic breast cancers with the BBB. Certain miRNAs and serpins are regulatory molecules in defining the metastatic potential of breast cancers. Targeting these factors that favor the metastatic microenvironment may provide future therapeutic interventions for the brain metastasis of breast cancers.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
Total thyroidectomy and adjuvant RIT followed by a suppressive dose of levothyroxine are the established therapeutic procedures of choice for DTC. The treatment of DTC has changed from a one size fits all standard to a more individualized approach. The use of less complete surgery as well as decision to use RIT and the dose administered are to be considered carefully in the treatment of DTC. Surveillance for very low risk DTC is an acceptable option. The aim to lower morbidity, lower the cost of treatment and improve patient quality of life is attainable using these principles.
The study aims to assess the predictive values of certain psychological factors on the quality of life in patients with Head and Neck Cancer after radiotherapy. The authors conclude that the identification and the understanding of the depressive symptoms of patients, their beliefs about their illness as well as their coping strategies may provide the basis for timely implementation of appropriate intervention that may improve the quality of life in patients.
This article pioneers the first electrophysiological evidence of vocalis muscle reinnervation following recurrent laryngeal nerve (RLN) repair in humans, marking a significant advancement in nerve repair science. By utilizing intraoperative nerve monitoring, the study confirms successful reinnervation through clear electromyographic responses, establishing a critical benchmark in RLN repair validation. This research is crucial for medical professionals as it highlights the importance of precise surgical techniques and rigorous postoperative monitoring, promising enhanced recovery and improved vocal cord function. The findings offer a fresh perspective on nerve regeneration, providing renewed hope for patients suffering from vocal cord paralysis. This study is essential reading for its innovative approach and its potential to reshape surgical and diagnostic practices. It engages readers by blending scientific rigor with a compelling narrative of medical advancement and patient hope.
The resolution of the photos added in the manuscript is quite low. High-resolution photos are required. Please submit the revision with high resolution photos.
ResponseHigh resolution photos have been submitted in the revised manuscript files.
Devianti MS, Mukarramah DA, Rini IS, Budiluhur A, Karsono R. Modalities for chest wall reconstruction following cancer ablation: A single center experience. Int Microsurg J 2019;3(2):5. https://doi.org/10.24983/scitemed.imj.2019.00117