Free tissue transfer is considered to be the gold standard of treatment for complex defects in various parts of the body. The increased use of smartphones and cellular technology in recent years has contributed to a revolutionary era in flap monitoring technology. Smartphone thermal imaging provides a low-cost alternative to traditional flap monitoring techniques. Nevertheless, there are significant concerns about the accuracy and reliability of this technique. In this article, we demonstrate three cases in which smartphone thermal imaging information was misleading. Within this analysis, we describe how clinical decision making should be approached in each of the cases. For a clinician to gain a comprehensive understanding of the limitations and capabilities of smartphone thermal imaging, it is essential to communicate clinically relevant events during the application of the technology.
For complex defects in various parts of the body, free tissue transfer has become the standard of care in the current era of reconstructive medicine. The success rate for free flap transfers has been reported to be as high as 95% [1]. In cases where the flap becomes compromised postoperatively, immediate intervention might be effective in preserving the flap, provided that it is performed in a prompt and timely manner. Therefore, the postoperative monitoring of the patient is one of the most critical steps in ensuring the success of free flap surgery [3].
The monitoring of flaps may be performed by a variety of techniques, such as superficial Doppler, implantable Doppler, infrared spectroscopy, laser Doppler, indocyanine green, fluorometer, as well as the older methods of tissue pricking, temperature monitoring, and clinical evaluation [4]. Recent advances in smartphone and cellular technologies have led to a breakthrough era of promising techniques for monitoring flaps [5]. There is growing use of smartphone thermal imaging (SPTI) as a tool to detect perforators during the flap planning process and to monitor the flaps postoperatively. With the advent of low-cost devices, real-time thermal imaging is now widely available and should be used in conjunction with existing technologies in order to provide additional clinical information that can be used to facilitate assessment, execution, and postoperative monitoring of each step of the tissue transfer procedure [6].
As a step toward successfully implementing the newly developed flap monitoring technology, it is imperative that users become familiar with the possible clinical outcomes of the device in order to gain a clear understanding of its limitations and capabilities. However, there have been relatively few studies examining flap monitoring techniques from a clinical standpoint. There is a need for further research into clinically and practically relevant parameters, such as flap salvage rate, false positive rate, and cost-effectiveness, in order to allow objective comparisons between monitoring techniques [7]. Nevertheless, we recognize that conducting a randomized clinical trial would be a difficult and costly endeavor due to the fact that a large sample size is needed since the failure rate is expected to be 5%. Meanwhile, flap monitoring is typically performed with a multimodal approach, and the flap monitoring protocol also varies considerably based on the preferences of each surgeon. Taking into account the issues raised above, there may be some concerns regarding the accuracy and reliability of this new technology for monitoring flaps.
Throughout the article, we present three cases of ambiguous observations that occurred during the application of SPTI for the postoperative evaluation of flaps. A false positive result may lead to unnecessary exploration of the flap, which could result in significant morbidity and medical expense. In contrast, false negative results can also contribute to delayed diagnosis, which in turn can result in flap loss. We analyze the phenomenon of ambiguous observations made during the use of SPTI to monitor flaps postoperatively in our discussion section.
Monitoring of the flap was performed using a FLIR ONE PRO camera (Extech, Nashua, NH, USA) in the first and second cases. In the third case, we monitored the flap using a SEEK Compact camera (SEEK thermal, Santa Barbara, CA, USA). Both the FLIR ONE PRO camera and the SEEK Compact camera produce a digital image which is superimposed with a thermal image. The FLIR ONE PRO camera has a thermal resolution of 160 x 20 and a temperature range of -4 °F to 752 °F [8]. The SEEK Compact camera is equipped with a 206 x 156 thermal sensor that can measure objects at temperatures ranging from -40 F° to 626 °F [9]. We may utilize either camera depending on its availability. A note of caution should be made regarding the fact that neither of the cameras was originally designed for medical applications.
At the end of the surgery, flap monitoring with SPTI was initiated. The flap was monitored every two hours during the first 48 hours following surgery, and then every four hours on the third day. The patients were discharged on the fourth postoperative day, and weekly office visits were arranged. A temperature measurement was conducted approximately 30 cm away from the patient's body. Gauze, clothing, and bandages were removed from the patient for a period of 5 minutes before any measurements were taken. A dry gauze was used to clean the flap and adjacent tissues in order to remove any sweat or fluid. A semi-quantitative comparison was conducted by using a color paddle to compare the thermal readings from the flap and adjacent tissues.
Case 1
This case report describes the surgical management of an 82-year-old male patient with basal cell carcinoma on the left supraclavicular fossa. The surgical interventions included the wide excision of the basal cell carcinoma, parotidectomy, clavicle excision, modified radical neck dissections, and resection of the clavicular head of the pectoralis major muscle and omohyoid muscle. An extensive surgical defect (40 cm in length by 14 cm in width) was created as a result of the resection of the tumor. Considering the considerable size of the defect, it was determined that a transverse rectus abdominus myocutaneous flap was inadequate to close the wound, particularly in the lateral region. In this immediate reconstruction, a free transverse rectus abdominus myocutaneous flap was performed along with the advancement of a deltopectoral flap. There was a small hematoma which was drained without the need for surgical intervention.
On the third postoperative day, SPTI showed a slight increase in temperature at both the caudal and cephalic edges, zone IV of the flap (Figure 1A). During the fourth postoperative day, the flap displayed a change in color and epidermolysis on its periphery (Figure 1B). As the central portion of the flap displayed normal clinical characteristics and an acceptable temperature according to the SPTI, it was determined that there was no need to re-explore the flap urgently in this case. Nonetheless, a partial flap necrosis was observed on the twentieth postoperative day (Figure 1C). As can be seen in Figure 1, the final area of necrosis corresponds to the areas that experienced a temperature change during the early follow-up period. Necrosis of the wound was treated as an expectant condition. Debridement was completed followed by the re-advancement of the deltopectoral flap and grafting of skin to close the wound.
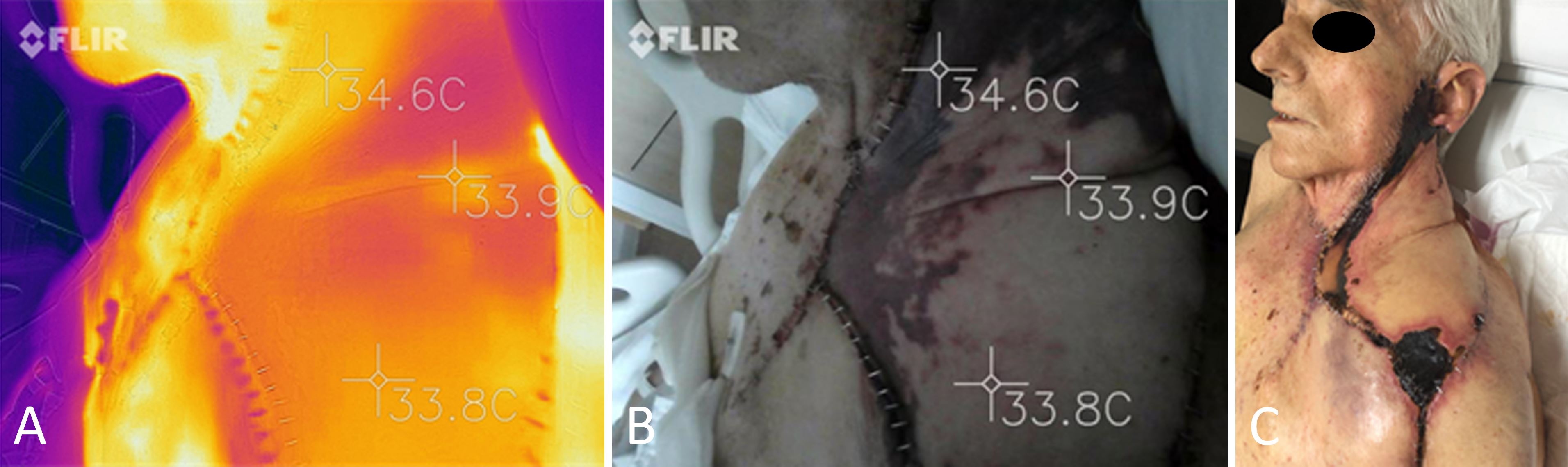
Figure 1. Reconstruction of the neck using a large transverse rectus abdominus myocutaneous flap. (A) An image of the transverse rectus abdominus myocutaneous flap captured with a FLIR ONE PRO camera on the third postoperative day indicates a slight increase in temperature at the flap's perimeter. (B) A clinical image depicting epidermolysis on the periphery of the flap on the fourth postoperative day. (C) Partial flap necrosis observed on the twentieth postoperative day. It can be observed that the final area of necrosis corresponds to the areas which had a temperature change during early follow-up.
Case 2
This case describes the treatment of a 57-year-old female patient who underwent immediate breast reconstruction using a microvascular breast sharing technique following a skin-sparing mastectomy (Figure 2A). The intraoperative indocyanine green angiogram confirmed that the flap received adequate blood supply (Figure 2B). The flap was based on the lateral thoracic pedicle.
Three days following surgery, the flap began to show mild signs of congestion (Figure 2C). However, the indocyanine green angiography revealed that the flap had normal perfusion. According to the SPTI, the flap was at an appropriate temperature compared to the surrounding skin (Figure 2D). Due to indocyanine green angiography and SPTI, there was a false sense of security created, which prevented an urgent investigation of the flap from being conducted. After a third week of follow-up, the flap developed fat necrosis and was ultimately reabsorbed (Figure 2E).
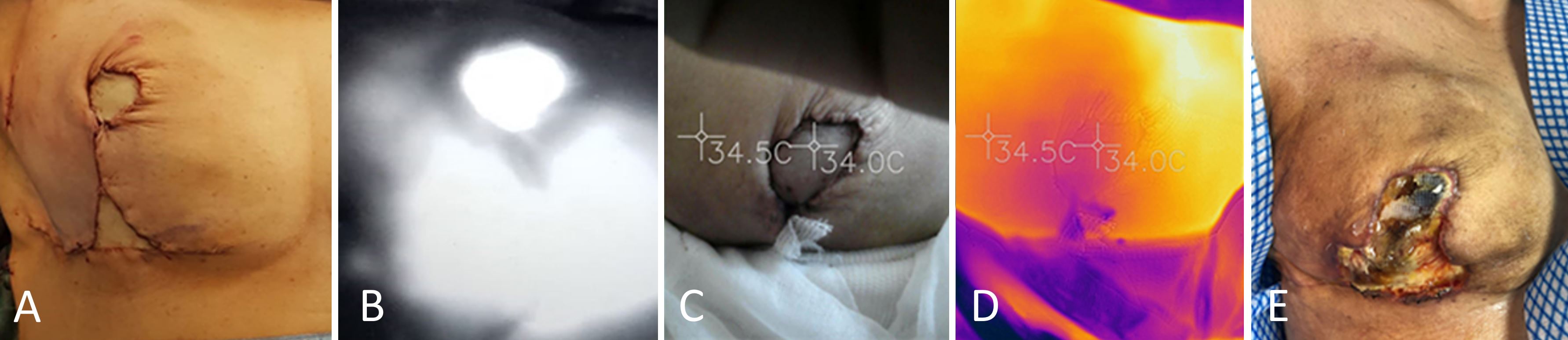
Figure 2. An immediate reconstruction of the breast using a free flap based on the contralateral lateral thoracic artery. (A) An immediate postoperative result after breast reconstruction using the breast sharing technique. (B) An intraoperative indocyanine green angiography showing adequate perfusion of the flap. (C) A clinical photograph of the flap taken three days after surgery shows the appearance of slight congestion. (D) A thermal imaging scan taken three days after surgery shows that the flap is at an appropriate temperature in comparison to the surrounding skin. (E) A third postoperative week examination reveals complete necrosis and dehiscence of the wound in the flap.
Case 3
A 76-year-old female patient underwent reconstructive surgery to repair a defect on her scalp with a free anterolateral thigh flap. Upon immediate postoperative assessment, the flap exhibited normal capillary filling, normal temperature, and normal color. Nevertheless, the SPTI result indicated a low temperature on the anterolateral thigh flap despite repeated calibration of the device (Figure 3A). As the flap exhibited excellent clinical characteristics (Figure 3B), it was not deemed necessary to re-explore it, and close clinical monitoring was maintained throughout the healing process. The patient was discharged from the hospital on the third postoperative day, and no flap complications were identified during follow-up (Figure 3C).
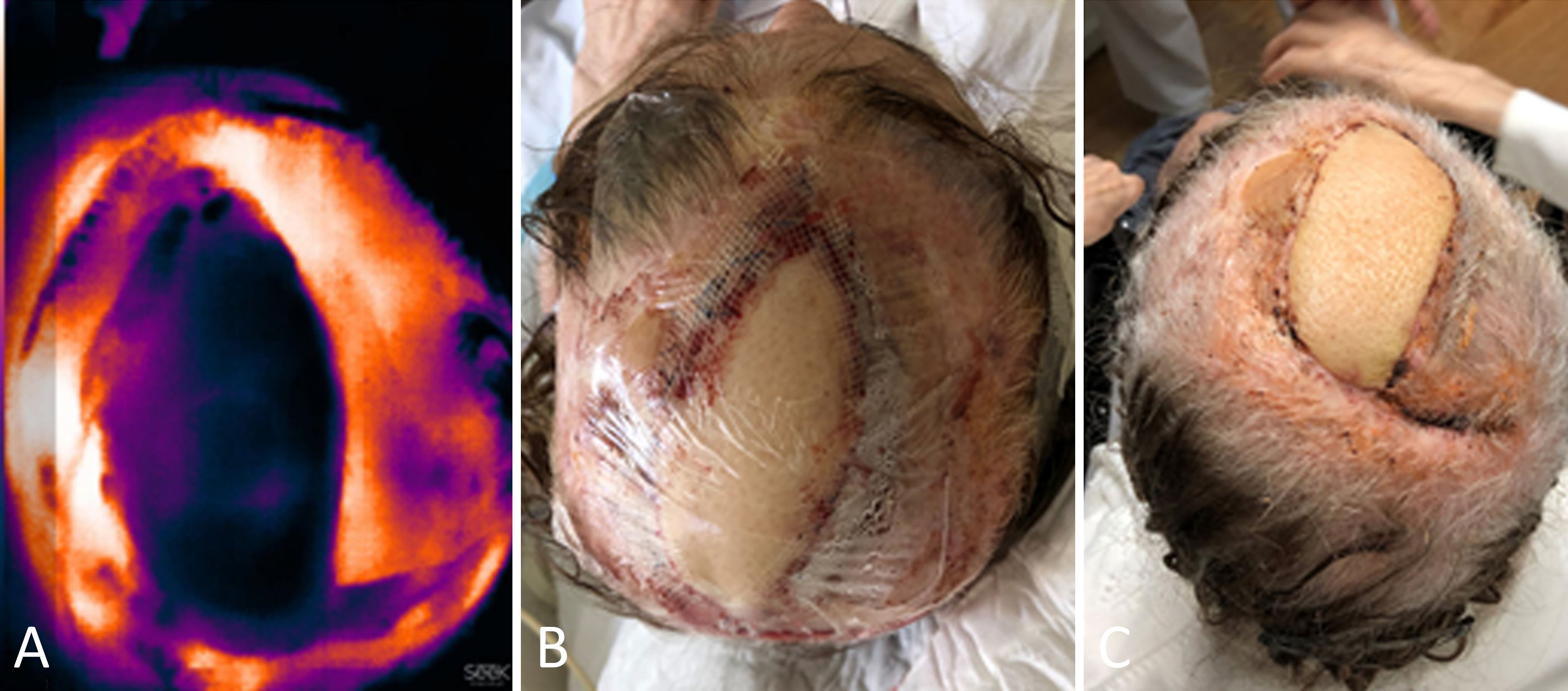
Figure 3. Reconstruction of the scalp with an immediate anterolateral thigh flap. (A) An image of the anterolateral thigh flap taken with a SEEK Compact camera at the end of the surgery indicates a marked decrease in the temperature of the flap. (B) An immediate postoperative photo of the anterolateral thigh flap showing appropriate clinical characteristics. (C) At twenty days postoperatively, the anterolateral thigh flap exhibits excellent clinical characteristics.
There are a variety of postoperative monitoring techniques available, all of which have different levels of complexity, invasiveness, and effectiveness. It is of paramount importance that the early detection of vascular compromise be conducted in the postoperative setting. The advent of thermal imaging has allowed non-contact vascular imaging to be performed indirectly without the use of ionizing radiation and intravenous contrast agents [6]. A major advantage of the procedure is its non-invasiveness as compared to more invasive methods.
As a thermal imaging device, SPTI has been developed to detect perforators during the planning process and to monitor flap status after surgery. The use of SPTI is an effective way to provide postoperative monitoring at a reasonable cost. Nonetheless, there remain concerns regarding the reliability and accuracy of its results for various reasons.
Firstly, the presence of skin temperature interference limits the application of thermographic imaging. In cases where a device has a lower resolution (and is generally less expensive), this factor is particularly influential [10]. There have been some suggestions for measuring the delta of temperature of the flap [11]. Particularly, this approach involves complex calculations, further complicating an already challenging situation. According to our experience, the most useful information can be obtained by comparing the flap temperature with that of the adjacent skin.
On the other hand, the temperature of both the flap as well as its adjacent tissue is affected by the temperature of the room. In contrast to smartphones, professional thermal cameras are more sensitive and thus are less susceptible to being misled by background thermal interference or artifacts [12]. Therefore, it is advisable to consider the effect of ambient temperatures on thermal monitoring of flaps in future experiments and clinical research.
SPTI is also limited by the fact that it relies upon a semi-objective interpretation of the color palette, which represents flap temperature [13]. It is possible that the clinically relevant thresholds and validity for STPI differ between models of the same manufacturer or between manufacturers [14]. Further investigation is needed to establish whether STPI is a reliable and reproducible technique to aid in free flap monitoring.
The first case involves the partial failure of a transverse rectus abdominus myocutaneous flap as a result of congestion in the peripheral part of the flap. In the beginning, SPTI simply displayed a slight increase in temperature along the periphery of the flap (Figure 1A). Following this, clinical signs of venous compromise were noted in the same zone. It is imperative to note that, from a physiological standpoint, early total flap failure is different from partial flap failure. This is because early microvascular compromise is caused by pedicle issues, while partial failure is caused by poor flap design and reverse flow. It was deemed unnecessary to re-explore the flap urgently in this case because the central portion of the flap revealed normal clinical characteristics and an acceptable temperature according to the SPTI.
Free tissue failure is not an all-or-none occurrence. Expectant management may result in acceptable outcomes while avoiding unnecessary iatrogenic complications [15]. The presence of localized thermal changes in a flap does not necessarily justify revision surgery. Instead, such changes should raise questions regarding potential complications. Similar observations have also been documented in animal models [16]. Furthermore, venous obstruction can cause congestion, which may also lead to a transient increase in the temperature of the flap [17]. From our observations in the first case, we are inclined to speculate that the slight rise in temperature was an early sign of venous compromise. Regardless, it would be prudent to verify our observations before making any conclusions.
For the second case, there were no obvious signs of flap failure at the beginning. The venous outflow did not exhibit any early disturbances. The indocyanine green angiography showed that the flap had normal perfusion. There was no evidence that the flap had a significantly lower temperature than the adjacent skin based on the SPTI (Figure 2D). It was impossible to conduct an adequate investigation of the flap within a reasonable timeframe because SPTI and indocyanine green angiography created a false sense of security. Ultimately, the flap suffered a total necrosis within three weeks of the surgery.
The third case is an example of a false positive observation. While the SPTI result indicated a low temperature of the anterolateral thigh flap (Figure 3A), the flap exhibited excellent clinical characteristics (Figure 3B), and therefore re-exploration of the flap was deemed unnecessary. There is a possibility that the false positive observation was caused by the use of Tegaderm® (3M) pads. Furthermore, the surrounding skin was also covered by the pad, but the temperature was appropriate. This exacerbated the false positive observation. As an alternative to re-exploring the flap, an intensive monitoring strategy was implemented. It was fortunate that the flap turned out to be a satisfactory outcome (Figure 3C).
In a prospective study utilizing SPTI, it has been observed that viable flaps are capable of achieving temperatures up to 3°C higher or lower than surrounding tissue without affecting perfusion [18]. There was only one failing flap documented in the study because failing flaps are relatively uncommon in surgery. Another study demonstrated high sensitivity and specificity when using a delta of more than 2°C between the flap and surrounding skin for one measurement, but low predictability [19]. However, the assessment of two positive outcomes within a one-hour period resulted in a sensitivity of 93%, a specificity of 96%, a positive predictive value of 57%, and a negative predictive value of 99%. There remains a degree of uncertainty about the exact range of decreased perfusion that leads to the need for intervention.
One limitation of this study was the heterogeneity of the flaps. The flaps of interest, however, were all free flaps with a skin paddle that allowed surface thermal imaging analysis. An ideal surveillance method should allow different types of free flaps to be monitored. In the case of certain flap types, buried flaps, or intraoral reconstruction, special attention must be paid to surveillance. However, this type of example is not available in the article.
Currently, our practice measures postoperative outcomes based on clinical characteristics and SPTI when doubt arises. In addition, we perform a quantitative analysis for glucose measurements in accordance with Hara et al. [20]. A combination of these approaches could lead to a more accurate evaluation of results.
An indication for re-exploring a flap should be derived from a clinical construct rather than from an isolated observation. Advances in technologies have led to both convenience and complexity in clinical decision-making. Clinicians should be aware that the use of a newly adopted technology can be misleading, especially without objective and standardized parameters.
Received date: September 26, 2021
Accepted date: February 07, 2022
Published date: July 25, 2022
Francisco Javier López-Mendoza (https://orcid.org/0000-0001-9065-198X).
The manuscript has not been presented at any meetings on the topic.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The authors obtained permission from the participants in the human research prior to publishing their images or photographs.
This research has received no specific grant from any funding agency either in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest declared by either the authors or the contributors of this article, which is their intellectual property.
It should be noted that the opinions and statements expressed in this article are those of the respective author(s) and are not to be regarded as factual statements. These opinions and statements may not represent the views of their affiliated organizations, the publishing house, the editors, or any other reviewers since these are the sole opinion and statement of the author(s). The publisher does not guarantee or endorse any of the statements that are made by the manufacturer of any product discussed in this article, or any statements that are made by the author(s) in relation to the mentioned product.
© 2022 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). In accordance with accepted academic practice, anyone may use, distribute, or reproduce this material, so long as the original author(s), the copyright holder(s), and the original publication of this journal are credited, and this publication is cited as the original. To the extent permitted by these terms and conditions of license, this material may not be compiled, distributed, or reproduced in any manner that is inconsistent with those terms and conditions.
The senior author (Dr. Isao Koshima) designed a tibial osseo-periosteal (TOP) flap. TOP flap has a favorable anatomical position with a thin skin around it, hence it is a good option for an island flap. TOP flap can be used for various mild to moderately sized osteo-cutaneous defects with low morbidity. In this article, the authors describe their experience of the first reported cohort of TOP flaps in clinical practice.
The likelihood of donor site ischemia following the harvesting of a fibula flap is extremely low, but it is potentially lethal if it occurs. The authors describe a case of ischemia of the lower extremity following a free fibula harvest for head and neck reconstruction. The authors discuss preoperative, intraoperative, and postoperative strategies to assist in diagnosing and managing risks associated with free fibula flap harvesting in this paper.
This study introduces an advanced tubularized radial artery forearm flap (RAFF) technique, marking an enhancement over traditional methods in addressing complex nasal reconstructions. It integrates functional and aesthetic considerations through a structured, multi-stage reconstruction process, emphasizing the use of tubularized flaps. Key learning points include the detailed crafting of stable nasal passages, strategic use of costal cartilage for robust structural support, and tailored postoperative care with silicone splints. The tubularized RAFF technique not only optimizes patient outcomes and quality of life but also provides plastic surgeons with critical insights to refine their techniques in facial reconstruction. Indispensable for professionals in the field, this article enriches the understanding of sophisticated reconstructive challenges and solutions.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Radiation forms a vital part of neoadjuvant treatment in locally advanced rectal cancer (LARC) and recurrent rectal cancers. The adverse effects of radiation are well recognized; however, radiation-induced perforation at the tumour site is very rare and is poorly understood. A symptomatic rectal perforation requires an emergency surgical intervention. However, it may present silently and can give rise to suspicion of disease progression and/or residual disease on imaging. Authors present two cases of silent perforations. Both gave rise to a considerable diagnostic dilemma, which was resolved by careful evaluation with MRI.
This pilot study compares the agreement in diagnosis between scanned slides and conventional microscopy in both low and high magnifications.
The article discusses a complex case of a 51-year-old Chinese woman diagnosed with a pituitary neuroendocrine tumor in the clivus, characterized by its invasive nature and atypical symptoms, leading to diagnostic challenges between chordoma and chondrosarcoma. Achieving a correct diagnosis through a transsphenoidal biopsy enabled effective surgical removal of the tumor without complications. Highlighting the critical role of biopsy for accurate diagnosis, especially with atypical imaging, the study showcases the efficacy of minimally invasive transnasal endoscopic biopsy techniques. It emphasizes the importance of a multidisciplinary approach for optimal patient outcomes in complex pituitary tumors, underlining the need for vigilance and adaptability in managing such rare conditions. This contributes valuable insights to the medical field, particularly for neurosurgery, otorhinolaryngology, and endocrinology practitioners.
The article stands out by providing a comprehensive analysis of a rare case of a midline branchial cleft cyst, a significant contribution given that only three other cases have been documented in medical literature. This scarcity underscores the article's value, making it a crucial read for medical professionals. It not only highlights the diagnostic challenges and treatment strategies for such atypical presentations but also enriches the understanding of branchial cleft cysts beyond the common lateral neck occurrences. By including a detailed review and comparative analysis of these few reported cases, the article offers unique insights into the demographic, symptomatic, and anatomical variations of branchial cleft cysts. This focused analysis makes the article an indispensable resource for clinicians, surgeons, and students in the medical field, aiming to enhance diagnostic accuracy, inform clinical practice, and ultimately improve patient outcomes in dealing with complex and rare presentations of congenital anomalies.
This narrative review advances the management of radiation-induced dysphagia in head and neck cancer treatment by integrating advanced imaging, precision dosimetry, and structured rehabilitation. Beyond focusing solely on dysphagia-optimized intensity-modulated radiotherapy, it explores a variety of approaches: MRI and CT provide detailed anatomical insights, while proton therapy offers a less toxic alternative, and early rehabilitation preserves swallowing function. This holistic model prioritizes patient quality of life, detailing how techniques like Do-IMRT reduce radiation to critical structures like the pharyngeal constrictors and larynx, maintaining effectiveness. The review advocates a multidisciplinary approach, encouraging collaboration among oncologists, radiologists, and therapists to enhance long-term patient well-being and promoting further research to elevate care standards.
This article explores the transformative impact of artificial intelligence on healthcare, providing readers with insights into its role in enhancing diagnostics, treatment, and patient care. It highlights potential cost savings and promotes a proactive approach to health management. The piece underscores advancements in robot-assisted surgery and artificial intelligence-enhanced virtual nursing, alongside efficient data management in healthcare. It addresses challenges and ethical considerations in integrating artificial intelligence, emphasizing the need to maintain clinical skills and empathy. Beneficial for readers, the article advocates a balanced approach that melds technological innovation with foundational healthcare principles, aiding informed decision-making in evolving healthcare landscapes.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Moran-Romero MA, López-Mendoza FJ. Postoperative monitoring of free flaps using smartphone thermal imaging may lead to ambiguous results: Three case reports. Int Microsurg J 2022;6(1):4. https://doi.org/10.24983/scitemed.imj.2022.00163