Background: Psammomatous calcifications are commonly observed in papillary thyroid carcinoma as well as in the non-tumoral thyroid parenchyma. A leading hypothesis suggests that psammoma bodies are remnants of papillae that have thrombosed, necrosed, and calcified. However, their prognostic value has not been clearly delineated.
Objective: To determine whether non-tumoral psammoma bodies are associated with adverse histologic findings.
Methods: The pathology archives were searched between 2015-2021 for cases of classic type papillary thyroid carcinoma. Cases with unknown lymph node status were excluded from the analyses. Patient demographics and histologic information were extracted from the reports. Chi square tests and logistic regression analyses were utilized to determine statistical significance (P <0.05).
Results: Our study included 584 cases and found that patients with lymph node metastases had significantly higher rates of non-tumoral psammoma bodies (71.0% vs. 24.7%, P <0.00001), viable tumor within lymphatics (32.4% vs. 6.1%, P <0.00001), and extrathyroidal extension (43.4% vs. 15.1%, P <0.0001) than those without nodal disease. Moreover, the percentage of patients with nodal disease with non-tumoral psammoma bodies was higher than those without (71.5% vs. 25.2%, P <0.00001). Additionally, patients with nodal involvement and non-tumoral psammoma bodies had significantly higher rates of viable tumor emboli within lymphatic channels than patients with nodal disease and without non-tumoral psammoma bodies (39.4% vs 15.2%, P <0.0001).
Conclusion: These findings suggest that non-tumoral psammoma bodies may signify extensive lymphatic spread and more aggressive tumor growth in classic type papillary thyroid carcinoma. It is therefore important to note their presence in the final pathology report.
Though papillary thyroid carcinoma (PTC), the most common endocrine malignancy [1], has a mostly favorable clinical course, certain factors, including older age, male sex, larger size, and extrathyroidal extension (ETE), foretell a worse prognosis [2]. A common histologic finding in PTC is that of laminated calcified spheres known as psammoma bodies (PB). These calcifications are often seen within the tumor proper and can range from highly abundant and easily observable to rare and scattered. PB can also be identified within the non-tumoral thyroid parenchyma both in the affected as well as the unaffected thyroid lobe. To attempt to explain the origin of PB, a study in 1980 hypothesized that these calcifications represent thrombosis and subsequent tumor necrosis and calcification of neoplastic papillae within the tumor proper and in intralymphatic spaces [3], the latter of which would explain their presence within thyroid parenchyma remote from the primary neoplasm. Other propositions for PB formation include the secretion of factors by the tumor cells [4] as well as an intracellular mechanism whereby PB are released upon tumor cell death [5,6]. In terms of prognostic value, some evidence is available that PB are associated with ETE, lymph node metastases, occult microcarcinomas, and overall portend a higher risk for tumor recurrence [7-9]. Specifically, in one study, extra-tumoral PB were associated with a higher incidence of tumor multifocality, extrathyroidal extension, and nodal metastasis when compared to tumors with only intratumoral PB [8]. Additionally, the presence of non-tumoral PB was associated with an ipsilateral or contralateral tumor, including microcarcinomas, suggesting the identification of PB within non-neoplastic thyroid parenchyma may warrant entire thyroid submission even if thyroidectomy was performed for benign etiologies [9]. However, other work is contradictory and has not shown a significant association between the presence of PB and histologic evidence of poor prognosis or tumor recurrence [10].
As molecular techniques became more sophisticated, activating genetic mutations along the mitogen-activated protein kinase (MAPK) pathway were identified in PTC [11-13]. Mutations in BRAF are most commonly reported (40%) [14] followed by RET rearrangements (20-30%) [15], and then mutations in RAS (10-15%) [16]. Of these molecular categories, tumors with RET rearrangements demonstrate higher rates of PB as well as lymph node metastases. This suggests that there may be complex interplay among alterations in this signaling pathway, the presence of PB, and nodal disease [17].
Given this information and the somewhat contradictory evidence regarding the prognostic value of PB, we sought to more clearly identify whether PB, specifically in non-tumoral thyroid tissue, correlates with adverse histologic findings and nodal metastases in the classic variant of PTC.
Following approval by our Institutional Review Board, the pathology archives were searched between the years of 2015-2021 for cases of classic type PTC with and without non-tumoral PB. Classic type PTC was chosen as the purely follicular variant of PTC does not produce PB and other variants, such as tall cell, are inherently more aggressive regardless of the presence of PB. Lymph node status was determined based on both clinical and pathologic findings. Multiple board-certified pathologists analyzed the slides (8-micron thickness, hematoxylin and eosin stained). Psammoma bodies were defined as lamellated concentric calcifications identified histologically. Cases with unknown lymph node status were excluded from the analyses. Patient demographics and pertinent histologic information were extracted from the pathology reports. Hematoxylin and eosin stains were used to analyze all slides and a Podoplanin (D2-40) immunohistochemical stain was used to analyze select slides. Chi square tests were utilized to determine statistical significance (P <0.05). Multivariable logistic regression analysis was also performed using STATA software (P <0.05 is significant).
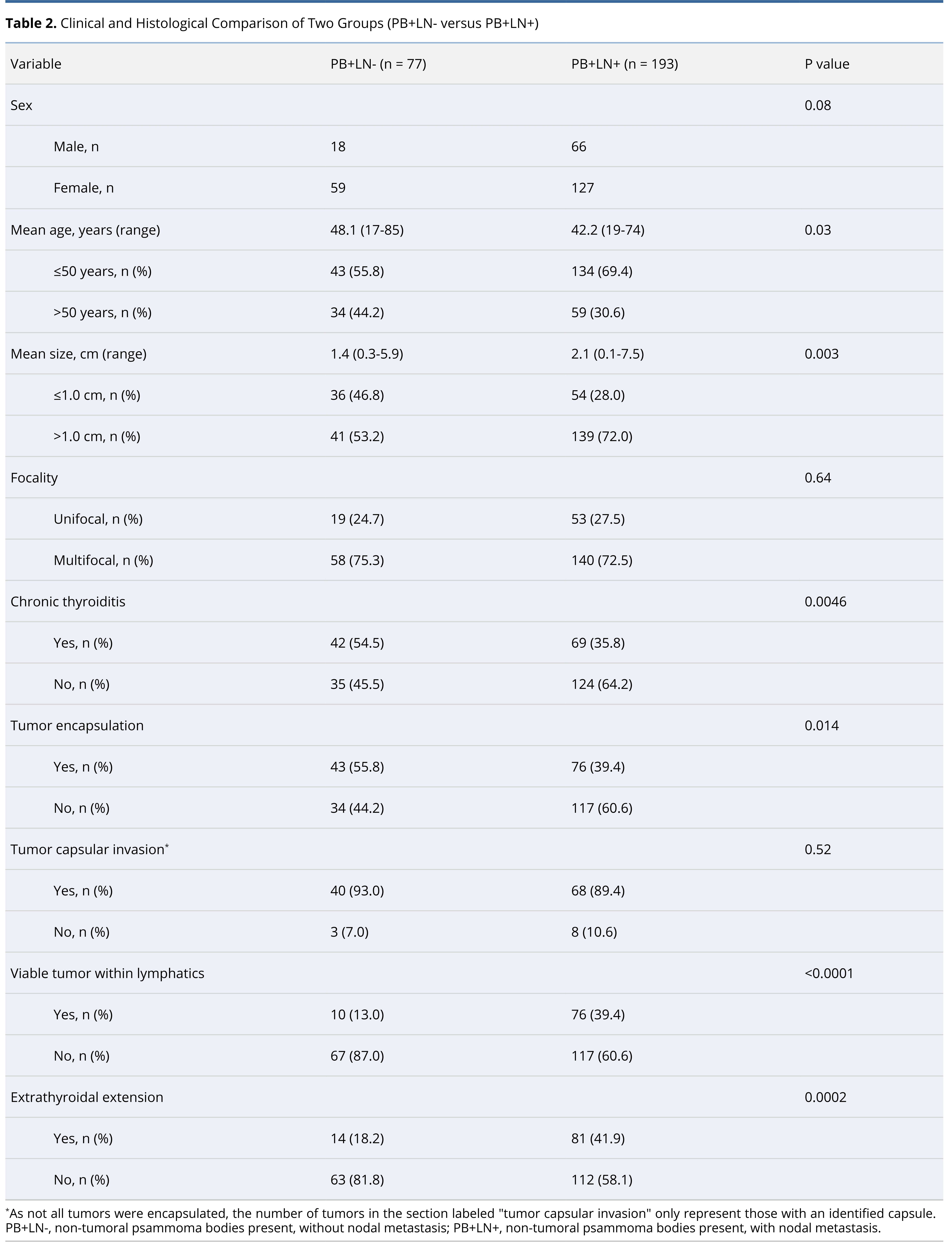
In total, 710 cases of classic type PTC were identified in our pathology archives between the years of 2015-2021. Of these cases, 126 did not have available corresponding lymph node data and were excluded from our analyses. Of the remaining cases, 272 had evidence of lymph node metastases, in both central and lateral compartments, while 312 had no evidence of nodal disease determined either by imaging or following histologic lymph node examination. Those with lymph node metastases had a significantly higher percentage of male patients (33.8% vs. 24.0%, P = 0.009), patients less than or equal to 50 years (65.8% vs. 55.4%, P = 0.01), non-tumoral psammoma bodies (71.0% vs. 24.7%, P <0.00001), viable tumor within lymphatics (32.4% vs. 6.1%, P <0.00001), and ETE (43.4% vs. 15.1%, P <0.00001). ETE was defined as microscopic or gross extension of disease outside of the thyroid capsule. These cases with lymph node metastases also demonstrated a lower incidence of chronic lymphocytic thyroiditis (34.9% vs. 45.2%, P = 0.01) and tumor encapsulation (40.8% vs. 50.0%, P = 0.03) when compared with cases without nodal disease. Table 1 provides a comprehensive overview of patient demographics and histological findings.
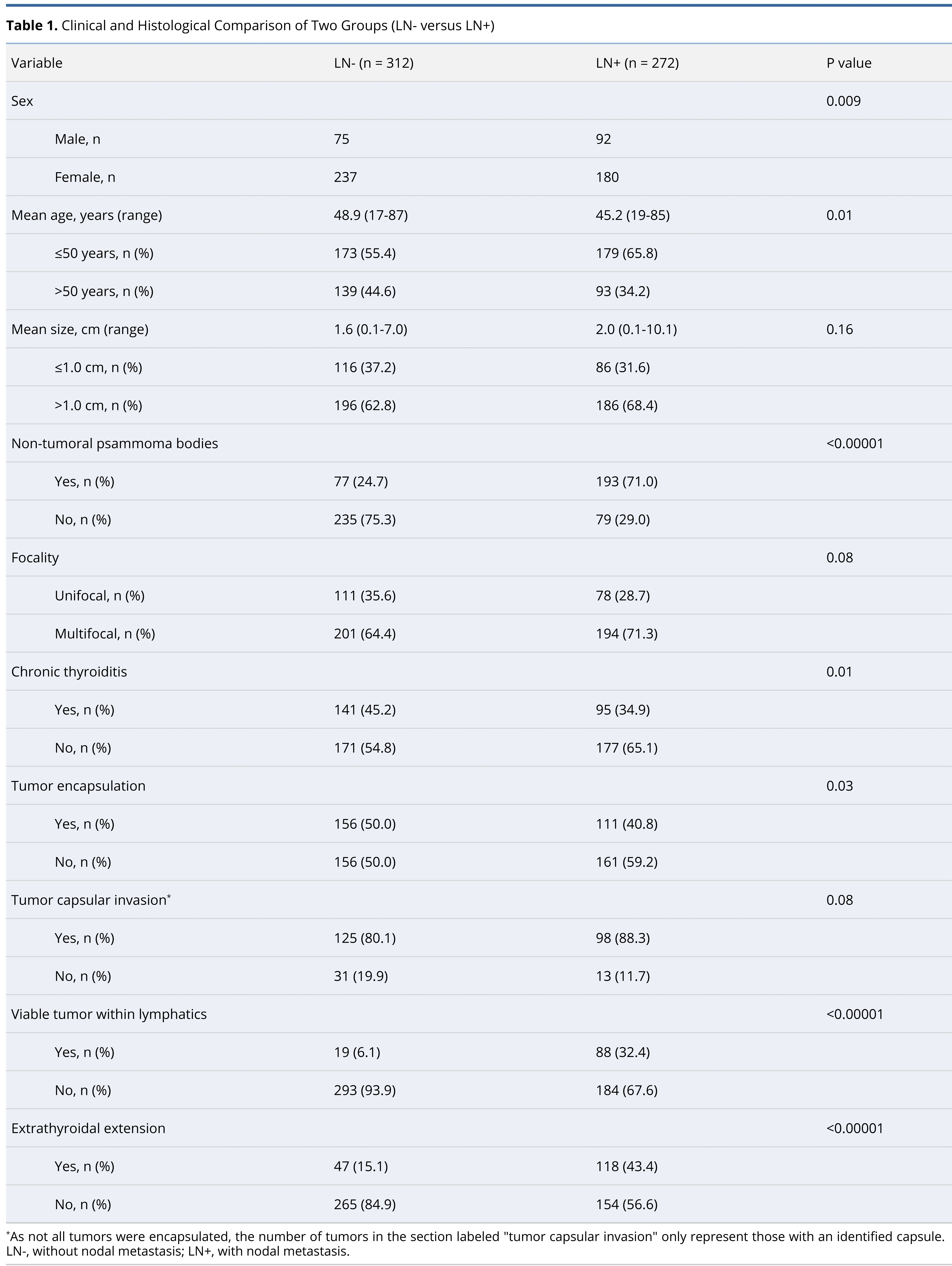
PB were present in non-tumoral thyroidal tissue in 270 of the cases. Of these specimens, 193 had associated lymph node metastases (PB+LN+ 71.5%) whereas 77 did not (PB+LN- 28.5%, P <0.00001). PB+LN+ cases demonstrated a significantly higher percentage of patients aged less than or equal to 50 years (69.4% vs. 55.8%, P = 0.03), tumors measuring >1.0 cm (72.0% vs. 53.2%, P = 0.003), viable tumor within lymphatics (39.4% vs. 13.0%, P <0.0001), and ETE (41.9% vs. 18.2%, P = 0.0002) than PB+LN- cases. Additionally, PB+LN+ cases showed lower rates of chronic thyroiditis (35.8% vs. 54.5%, P = 0.0046) and tumor encapsulation (39.4% vs. 55.8%, P = 0.014) than PB+LN- cases. Further details are provided in Table 2.
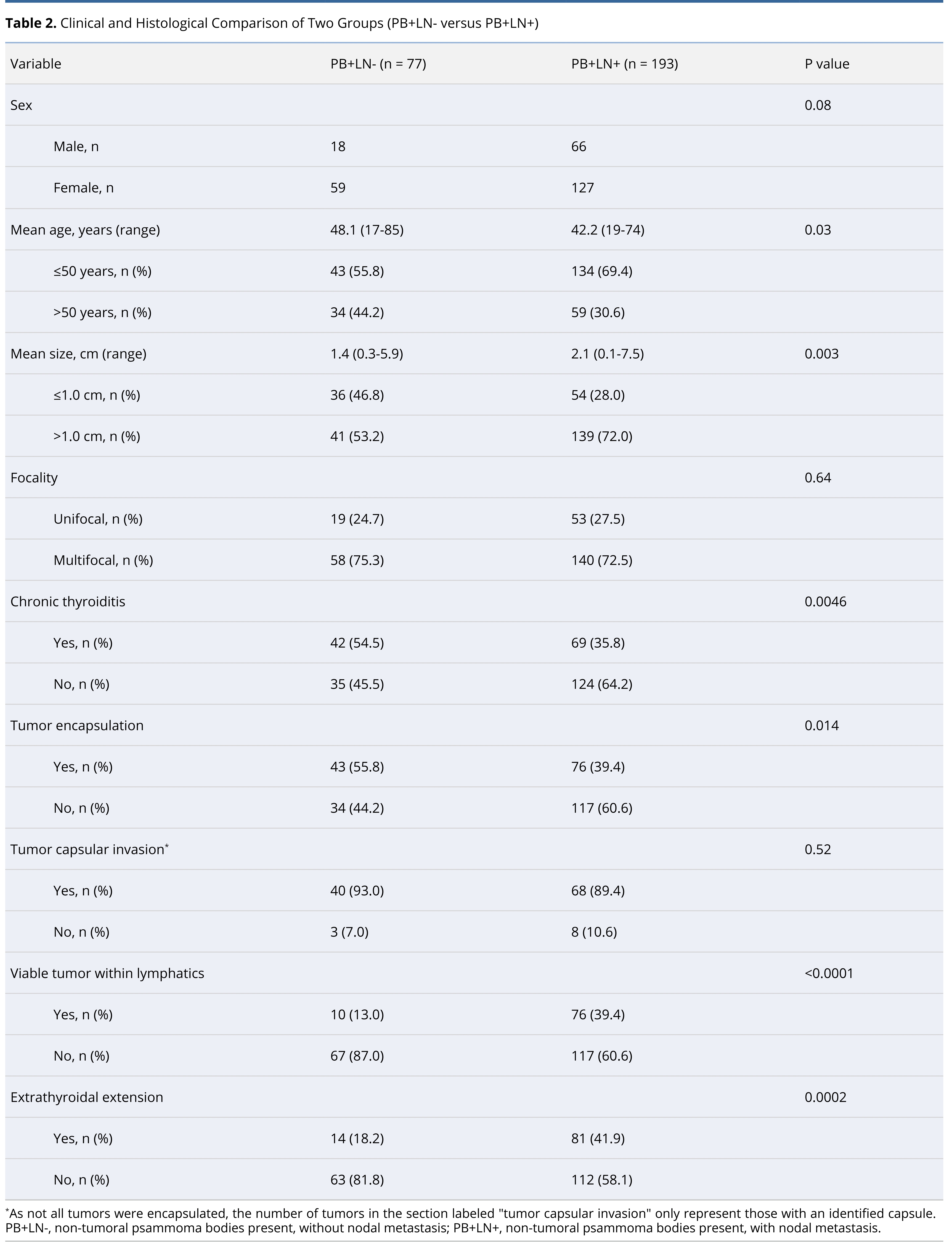
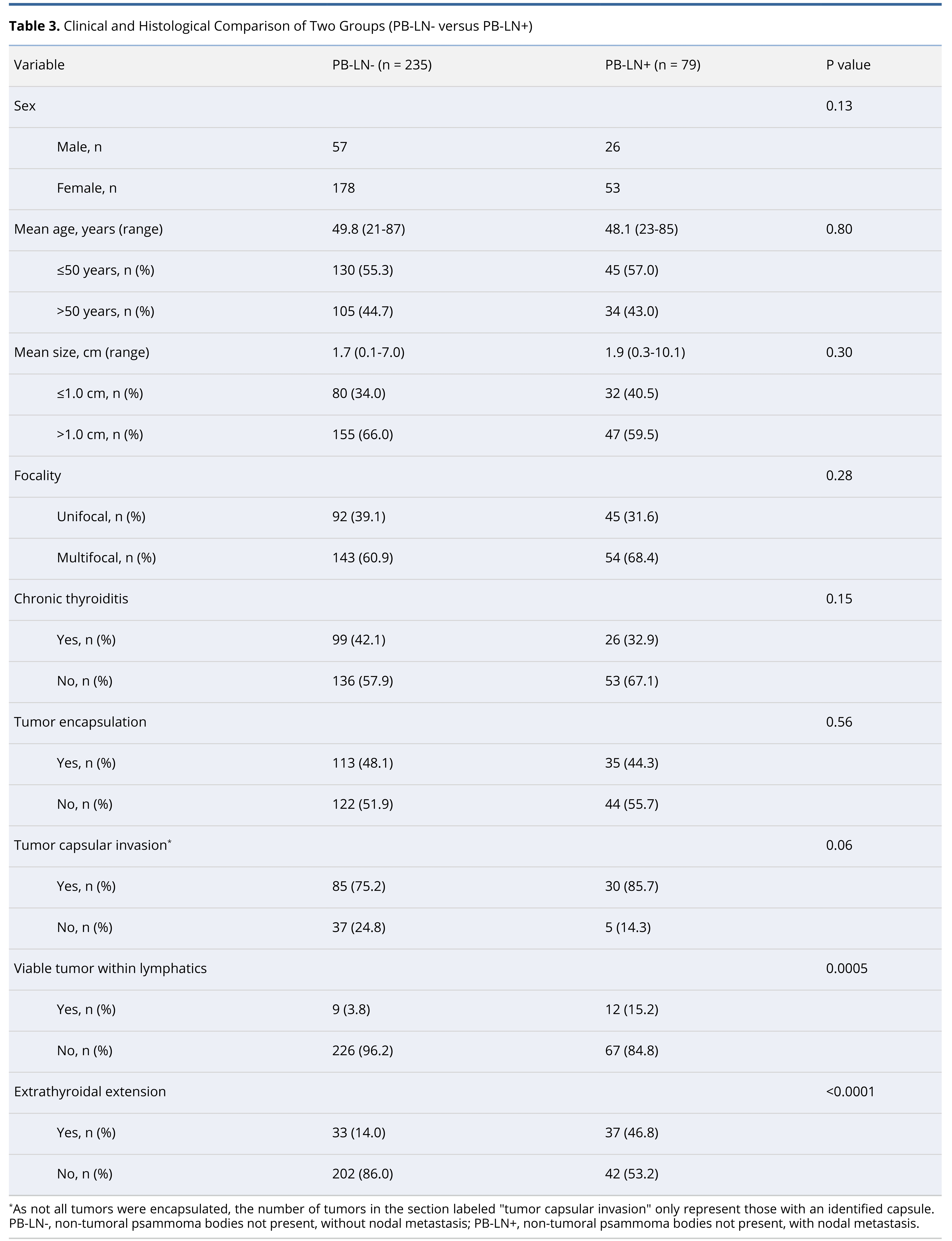
Three hundred and fourteen cases did not have identifiable PB in non-tumoral thyroidal tissue. Of these specimens, 79 had associated lymph node metastases (PB-LN+ 25.2%) whereas 235 did not (PB-LN- 74.8%). Of all parameters assessed, the only statistically significant findings were increased rates of viable tumor within lymphatics (15.2% vs. 3.8%, P = 0.0005) and ETE (46.8% vs. 14.0%, P <0.0001) in PB-LN+ cases vis-à-vis PB-LN- cases. Further details are provided in Table 3.
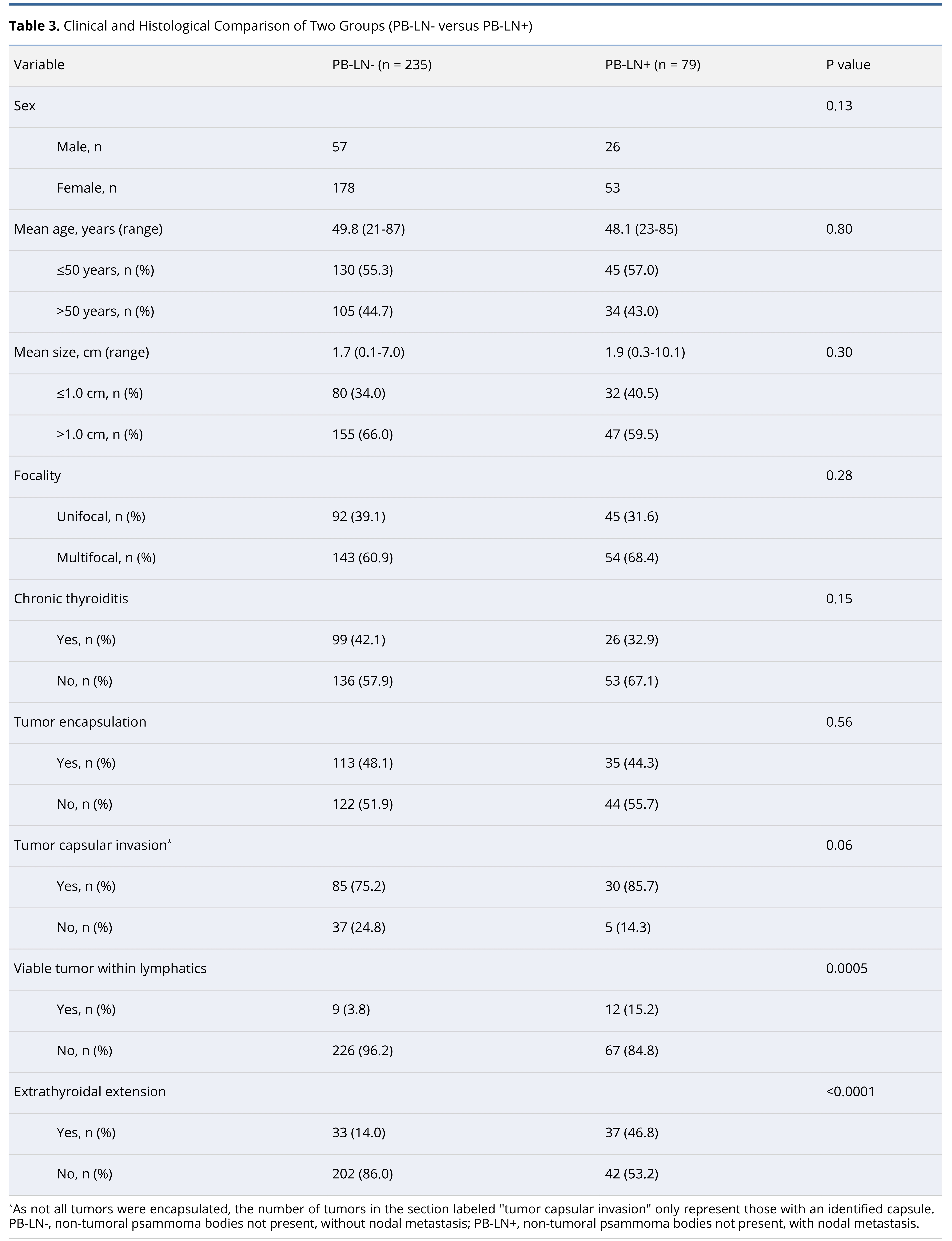
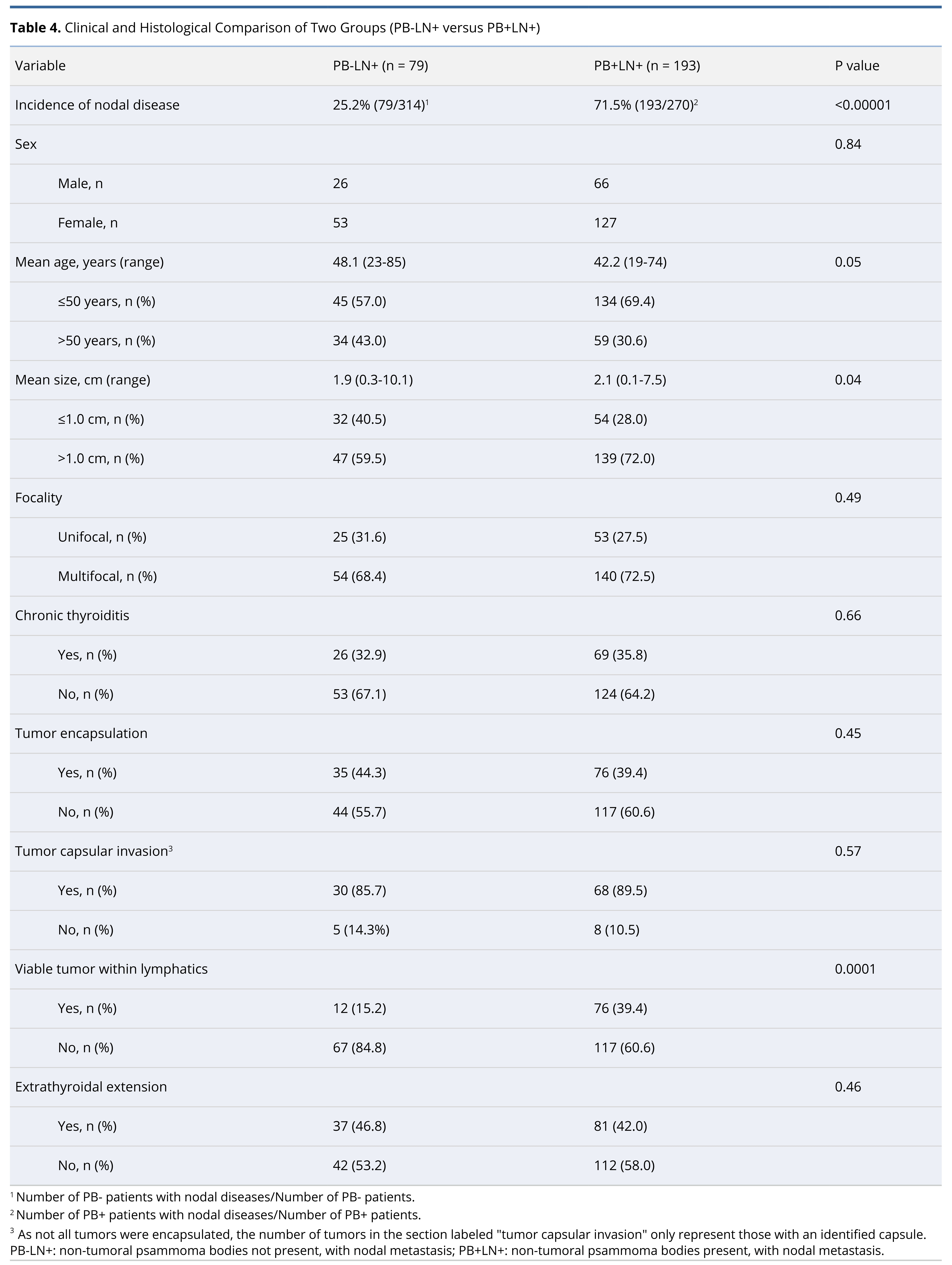
When comparing tumors with nodal disease, those with non-tumoral PB (PB+LN+) had significantly increased rates of lymph node metastases compared to those without non-tumoral PB (PB-LN+) (71.5% vs. 25.3% P <0.00001). No significant differences in extranodal extension were identified between groups (10/79, 12.7% vs. 34/193, 17.6%, P = 0.39). Correspondingly, PB+LN+ tumors had significantly higher rates of viable tumor within lymphatic spaces than PB-LN+ tumors (39.4% vs. 15.2%, P = 0.0001). No significant differences were noted between PB+LN+ and PB-LN+ in terms of male:female ratio (52.0% vs. 49.1%, P = 0.84), multifocality (72.5% vs. 68.4%, P = 0.49), presence of chronic thyroiditis (35.8% vs. 32.9%, P = 0.66), tumor encapsulation (39.4% vs. 44.3%, P = 0.45), tumor capsular invasion (89.5% vs. 85.7%, P = 0.57), and ETE (42.0% vs. 46.8%, P = 0.46). Further details are provided in Table 4.
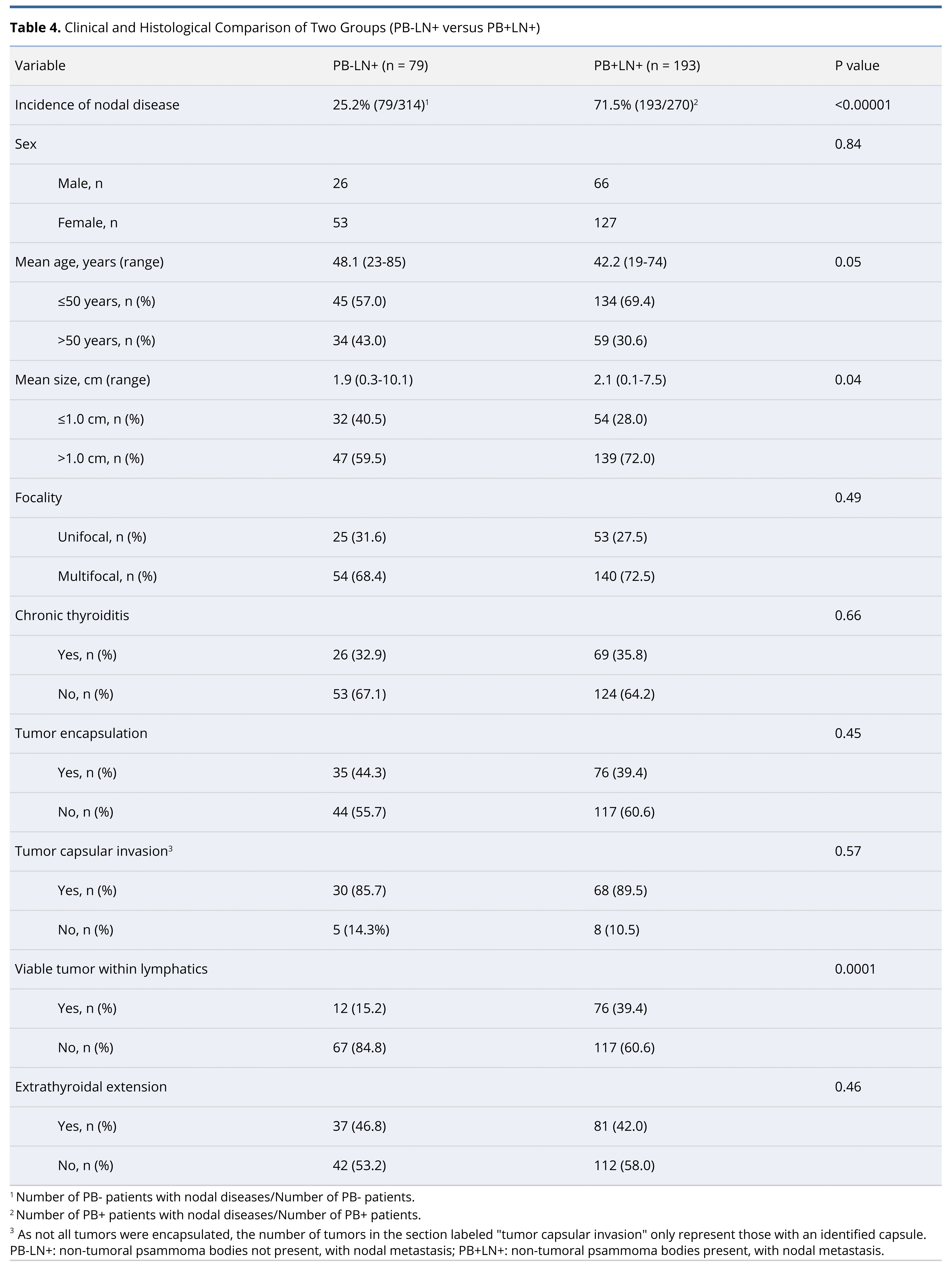
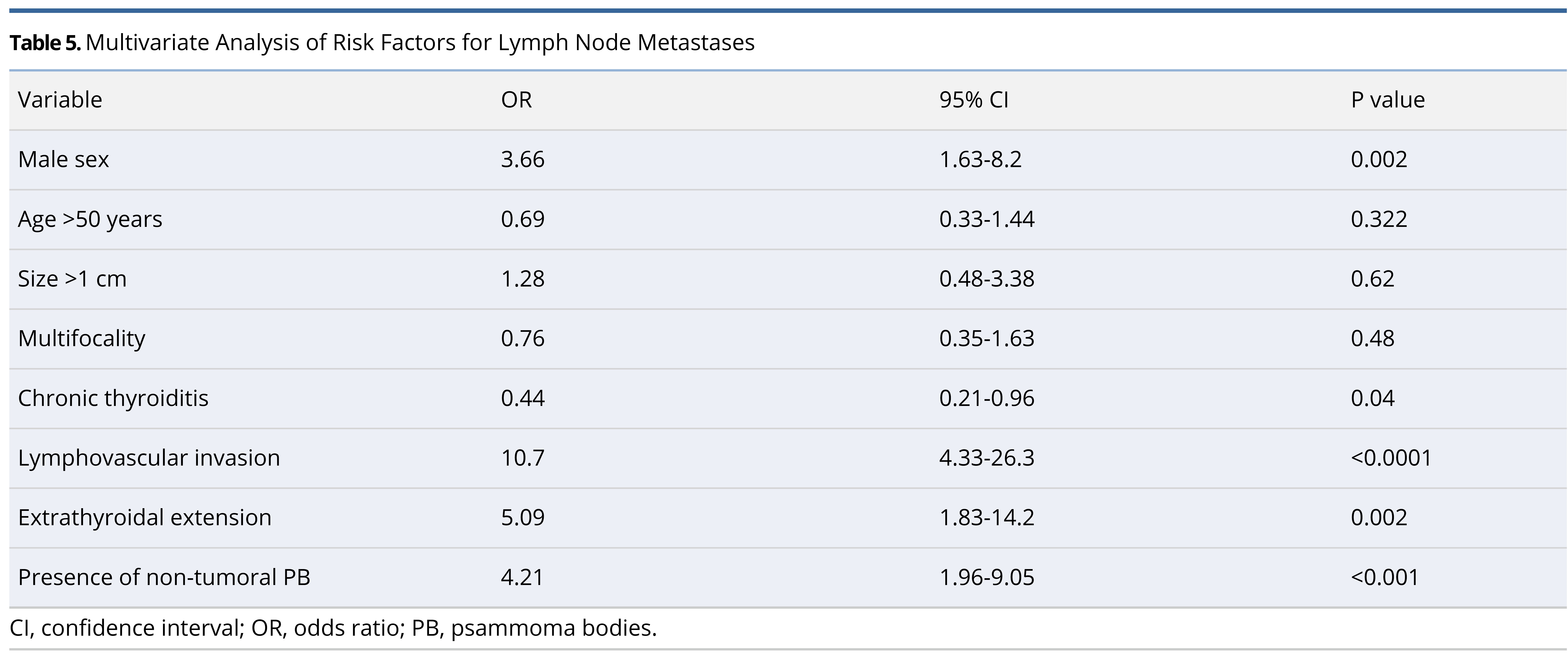
Additionally, we performed a logistic regression analysis to determine the effects of multiple variables on lymph node metastasis (Table 5). We found that multiple factors were significantly associated with nodal disease including male sex (P = 0.002), lack of chronic thyroiditis (P = 0.04), lymphovascular invasion (P <0.0001), extrathyroidal extension (P = 0.002), and the presence of non-tumoral psammoma bodies (P <0.001). Furthermore, we assessed the location of non-tumoral PB and found that they were located within lymphatic channels (Figure 1).
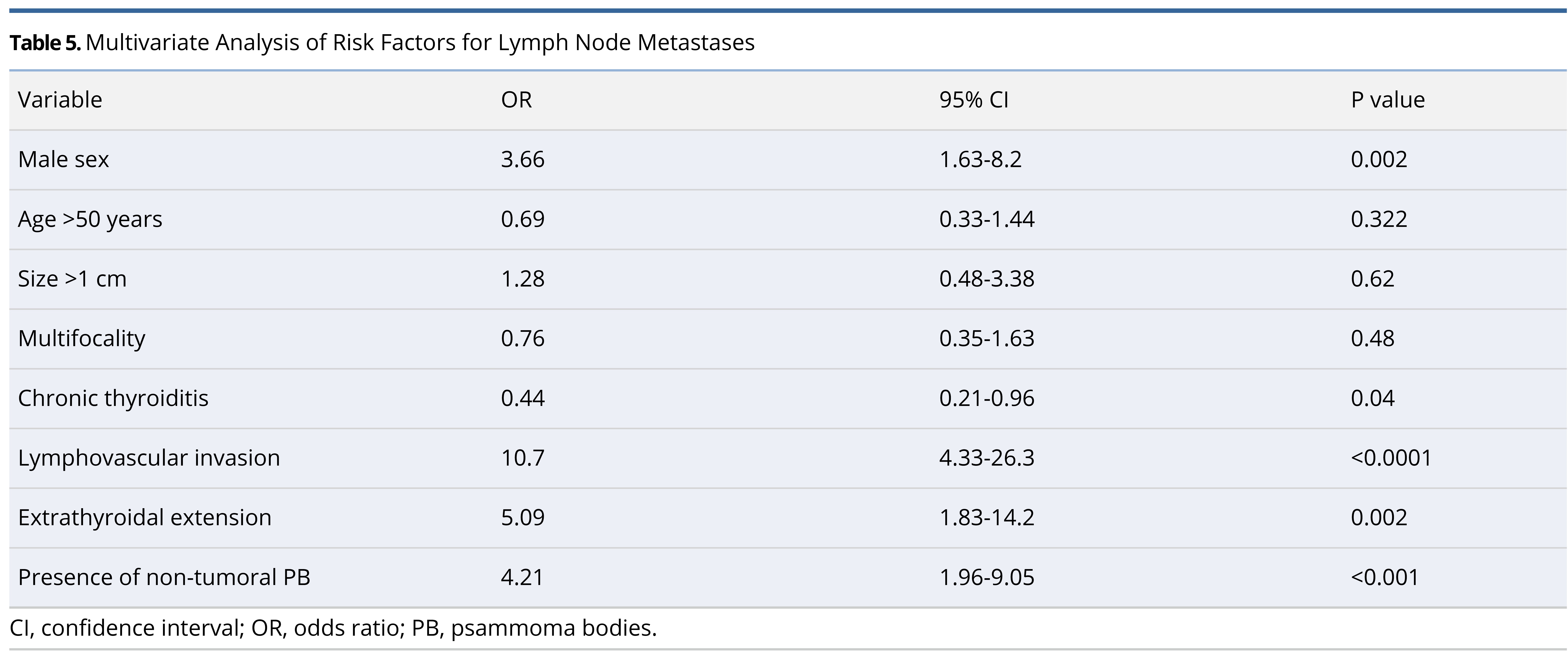
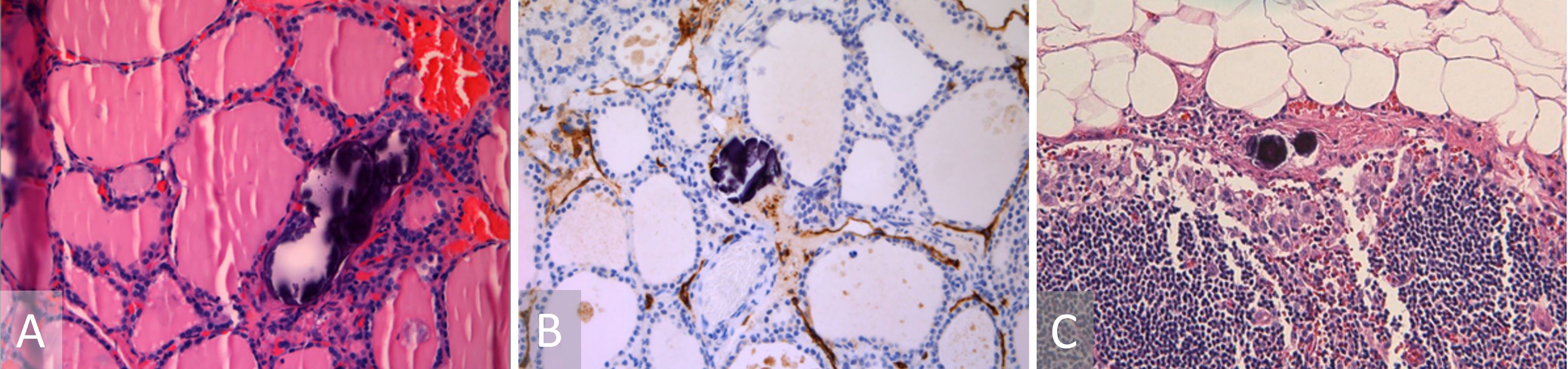
Figure 1. (A) Hematoxylin and eosin stain depicting a non-tumoral psammoma body. (B) Podoplanin (D2-D40) immunostain highlighting a lymphatic channel encasing a psammoma body. (C) Lymph node with psammoma bodies in the subcapsular lymphatic space.
In our study, patients with classic variant PTC with lymph node metastases were significantly more likely to be male, of younger age (≤50 years), have lower rates of background chronic thyroiditis, have infiltrative rather than encapsulated tumors, and demonstrate higher rates of viable tumor within lymphatics as well as ETE. These patients with nodal disease were also significantly more likely to have non-tumoral PB than patients without lymph node metastases. When further stratified into groups with and without non-tumoral PB and with and without lymph node metastases, our study revealed that nodal disease is more frequently found in tumors with higher rates of viable tumor within lymphatics and ETE independent of the presence of non-tumoral PB. However, when comparing only patients with nodal disease, we found that the overwhelming majority of tumors with nodal involvement demonstrated non-tumoral psammoma bodies. Additionally, though viable tumor within lymphatics and ETE are independent histologic factors associated with lymph node metastases, we also illustrated that the majority of tumors with non-tumoral PB had significantly higher rates of intralymphatic tumor emboli and a higher percentage of lymph node metastases than those without non-tumoral PB. Moreover, our multivariate analysis also suggests an important link among lymphovascular invasion, extrathyroidal extension, and the presence of psammoma bodies with nodal disease. Even though our study shows the likelihood that a papillary thyroid carcinoma will spread to lymph nodes is multifactorial, the presence of non-tumoral psammoma bodies is strongly associated with these other poor prognostic factors. Therefore, we emphasize its importance with regard to severity of disease.
A question that often arises in the discussion of non-tumoral PB is whether non-tumoral PB are truly remnants of viable tumor within lymphatics and not simply multifocal regions of tumor that have calcified. In our extensive literature search to identify a correlation between PB and tumor multifocality, we found that most studies include information regarding tumor multifocality and PB status as it pertains to lymph node metastases and very few actually compare tumors with non-tumoral PB to those without. Our extensive literature search yielded only one paper which made this direct comparison and did show that PB were significantly associated with tumor multifocality [8]. However, we did not find any association between non-tumoral PB and tumor multifocality in our large study. One possible explanation may be the definition of “multifocality” and the inter-observer variability in calling small tumor deposits as true multifocal lesions versus a pocket of lymphatic invasion by tumor. Thus, we support the hypothesis that PB may be “ghosts” of papillae past, including tumor that has invaded into lymphatic spaces, that have thrombosed, necrosed, and consequently calcified rather than discrete multifocal tumor deposits [3] as after careful histologic and immunohistochemical analysis, we confirmed that non-tumoral PB are located within lymphatic spaces in the non-tumoral thyroid parenchyma rather than deposited either within viable tumorlets or within non-neoplastic thyroid follicles. Additionally, we must note that in this study we did not specifically evaluate PB within the primary tumor (the majority of cases of classic PTC have them) nor did we correlate their presence or absence with those present within the lymphatics of non-tumoral thyroid parenchyma. The intra-tumoral PB are usually encountered in the stroma, within the papillae, or within the foci of cystic change and not within the lymphatics. Our study specifically focused on the intralymphatic spaces containing PB within the non-tumoral thyroid parenchyma to support the hypothesis that tumors with this feature tend to have more aggressive features i.e., nodal metastases. Moreover, we cannot identify an alternative mechanism besides calcification of tumor papillae for the presence of PB in non-tumoral thyroid as the thyroid parenchyma surrounding these structures is histologically unremarkable and thus should not be a source for their formation. Taken together, these findings suggest non-tumoral PB act as a surrogate marker for invasive behavior.
While PB are commonly associated with PTC, these calcifications can also be observed in a variety of other tumor types including but not limited to serous ovarian carcinoma [18], lung adenocarcinoma [19], and meningiomas [20]. As in the thyroid, the mechanism by which they form is still poorly understood. However, the chemical composition of PB is fairly conserved across these tumor types and is typically calcium phosphate [21]. PB have also been found to be associated with cellular debris and tumor necrosis [3,21], and ultrastructural studies have corroborated this dystrophic mechanism for PB formation, supporting the hypothesis that the involution of papillae is the most likely source [22,23].
Despite their common occurrence in many papillary tumor types and detailed studies regarding their composition, no conclusive verdict has been reached as of yet regarding the utility of PB in terms of patient prognosis. For example, in serous ovarian carcinoma, the identification of PB was associated with increased tumor apoptosis and longer survival times [18]. In a similar vein, lung adenocarcinomas with PB tend to have EGFR mutations, which are more susceptible to targeted therapies [19]. In PTC, there is some evidence suggesting PB are associated with more aggressive disease [7-9]. These findings taken together with our large comprehensive study add further support for the idea that the presence of PB, particularly in non-tumoral parenchyma, may signify a more aggressive and infiltrative tumor with a higher propensity to involve lymphatic spaces and metastasize to lymph nodes.
Taken together, we have shown that classic variant PTC with non-tumoral PB have significantly higher rates of viable tumor within lymphatic spaces than tumors without non-tumoral PB and are more closely associated with nodal metastases than tumors without non-tumoral PB. We do acknowledge that one limitation of this study is its retrospective nature. Given this information, we believe it is vital to document the presence of PB in non-tumoral thyroid parenchyma as such tumors may behave in a more aggressive fashion and the ultimate prognostic implications of these findings will require additional studies with long term follow-up.
Received date: December 08, 2021
Accepted date: January 20, 2022
Published date: February 16, 2022
All authors contributed to the conception and design of the study. The first draft of the manuscript was written by Maria A. Gubbiotti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data were presented at the American Thyroid Association Meeting September 2021 (Virtual).
Ethics approval was obtained from the Hospital of the University of Pennsylvania. The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This research has received no specific grant from any funding agency either in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest declared by either the authors or the contributors of this article, which is their intellectual property.
It is pertinent to note that all opinions and statements made by the author(s) throughout this article are solely those of the author(s). They may not be representative of those of their affiliated organizations, the publishing house, editors, or other reviewers since they are the opinions and statements of the author(s) alone. The publisher does not guarantee or endorse any statements made by the manufacturer of any product mentioned in this article or the author's evaluation.
© 2022 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). In accordance with accepted academic practice, anyone may use, distribute, or reproduce this material, so long as the original author(s), the copyright holder(s), and the original publication of this journal are credited, and this publication is cited as the original. To the extent permitted by these terms and conditions of license, this material may not be compiled, distributed, or reproduced in any manner that is inconsistent with those terms and conditions.
This case reports a 40-year-old male patient with right sinonasal teratocarcinosarcoma. It is a rare malignant lesion with extremely aggressive and commonly recurrent nature. The average survival period is less than two years.
The author presents a case of BCC developed on an active lesion of mucocutaneous leishmaniasis as one of many cases of skin cancer frequently seen at the Regional Leishmaniasis Control Center (RLCC) clinics in Yemen.
Dysphagia is an important consequence of cancer treatment and has overarching implications on quality of life. Using the FOIS, we demonstrated that swallowing function may be worse in the long term in patients with OPSCC undergoing triple therapy, although this finding did not reach statistical significance. This study emphasizes the importance of diligent selection in patients undergoing TORS to avoid poor functional swallowing outcomes, particularly in those that may need adjuvant chemoradiation therapy. A study with a larger sample size may determine the significance of these trends.
This article holds critical relevance for healthcare professionals, particularly in the fields of microsurgery, oncology, and vascular medicine. It thoroughly examines the diagnostic challenges faced in distinguishing between recurrent lymphedema and deep vein thrombosis in elderly cancer patients following lymphovenous anastomosis surgery. It highlights the significant risk of misdiagnosing deep vein thrombosis as lymphedema, a mistake that can delay critical treatment due to their clinical similarities. The case study of a 79-year-old patient emphasizes the importance of a comprehensive reassessment, considering the patient's entire medical history, including the effects of cancer treatments like immunotherapy. The article stresses the need for a holistic approach to patient management and the utilization of advanced diagnostic tools for accurate diagnosis and treatment. It is essential reading for its insights into the complex dynamics of postoperative care and the critical importance of accurate diagnosis in treating elderly cancer patients effectively.
Clinicians should remain on high alert for oropharyngeal masses while evaluating chronic cough patients. When a mass lesion is observed in the tongue base, ectopic lingual thyroid must be considered in the differential diagnosis, and the diagnosis must be verified using USG and scintigraphy. It is important to pay more attention to a local irritation that is related to the increased physiological demand for thyroxine during puberty and pregnancy.
Authors present a case report of a patient with history of RYGB who underwent thyroidectomy and parathyroidectomy with postoperative development of severe recalcitrant hypocalcemia. Hypocalcemia was managed conservatively initially but subsequently required gastrostomy tube placement with successful resolution of hypocalcemia.
Total thyroidectomy and adjuvant RIT followed by a suppressive dose of levothyroxine are the established therapeutic procedures of choice for DTC. The treatment of DTC has changed from a one size fits all standard to a more individualized approach. The use of less complete surgery as well as decision to use RIT and the dose administered are to be considered carefully in the treatment of DTC. Surveillance for very low risk DTC is an acceptable option. The aim to lower morbidity, lower the cost of treatment and improve patient quality of life is attainable using these principles.
This article pioneers the first electrophysiological evidence of vocalis muscle reinnervation following recurrent laryngeal nerve (RLN) repair in humans, marking a significant advancement in nerve repair science. By utilizing intraoperative nerve monitoring, the study confirms successful reinnervation through clear electromyographic responses, establishing a critical benchmark in RLN repair validation. This research is crucial for medical professionals as it highlights the importance of precise surgical techniques and rigorous postoperative monitoring, promising enhanced recovery and improved vocal cord function. The findings offer a fresh perspective on nerve regeneration, providing renewed hope for patients suffering from vocal cord paralysis. This study is essential reading for its innovative approach and its potential to reshape surgical and diagnostic practices. It engages readers by blending scientific rigor with a compelling narrative of medical advancement and patient hope.
Gubbiotti MA, Baloch Z, Montone K, Livolsi V. Non-tumoral psammoma bodies correlate with adverse histology in classic type papillary thyroid carcinoma. Arch Otorhinolaryngol Head Neck Surg 2022;6(1):3. https://doi.org/10.24983/scitemed.aohns.2022.00156