Objective: This research illuminated the exploration of an alternative path in the therapeutic landscape of cholesteatoma, an abnormal growth in the ear that commonly necessitates surgical resolution. This study was motivated by the reality that not every patient is inclined, or has the capacity, to pursue surgical intervention due to personal reservations or geographic limitations. Consequently, our study highlights the utilization of 5-fluorouracil (5-FU), a compound acclaimed for its efficacy in oncology chemotherapy, as a potential substitute for invasive measures in cholesteatoma treatment. The main objective was to critically examine the non-invasive approach to managing cholesteatoma through 5-FU administration in ambulatory care environments.
Methods: This investigation was built upon a case series study, meticulously assessing the clinical outcomes of patients who underwent treatment for cholesteatoma in an ambulatory care facility. Between November 2007 and November 2020, 15 ears of 14 patients were evaluated for cholesteatoma in the external auditory canal, attic area, or previously operated mastoid cavity. After thorough ear cleaning and debris extraction, the obtained tissues were examined pathologically. The treatment regimen consisted of a monthly application of roughly 0.3 mg of 5% 5-FU cream (Kyowa Kirin) to the afflicted region. The efficacy of this therapy was gauged after a one-month duration using Takahashi's criteria, with the cholesteatoma's status meticulously documented monthly.
Results: Fourteen patients were monitored (average age of 78 years; 71% female) at our ambulatory facility for cholesteatoma diagnosis. Left ear cholesteatoma was diagnosed in nine patients (60%), right ear in four (27%), and bilateral in one (13%). Reported symptoms included hearing impairment (33%), otorrhea and otalgia (17% each), ear fullness (13%), ear discomfort (11%), and bleeding (6%). The cholesteatoma's location was distributed as follows: external auditory canal (67%), attic (20%), and mastoid cavities (13%). Clinical observations revealed 87% of patients with epithelial debris, and 13% with granulation and epithelial debris. Pathological examinations confirmed the diagnosis of cholesteatoma. No surgical interventions were performed. The clinical appraisals revealed 87% positive results, 13% satisfactory outcomes, and no cases of poor outcomes.
Conclusion: Our findings advocate the feasibility of conservative, non-surgical cholesteatoma treatment with topical 5-FU cream within an ambulatory care framework. This approach might serve as an effective alternative for certain patient demographics, including the elderly and individuals residing in remote areas where access to specialized medical services is limited. The adoption of such a treatment modality could potentially enhance cholesteatoma management, leading to improved quality of life for patients.
Patients presenting with cholesteatoma frequently report symptoms such as otorrhea, otalgia, and ear fullness [1]. Distinguishing cholesteatoma from cerumen or other debris solely based on clinical examination can be challenging, except for the presence of distinctive pearly epithelial debris. Consequently, a definitive diagnosis requires pathological examination. Traditionally, the management of cholesteatoma has predominantly occurred in ambulatory care settings, with subsequent referral of patients to hospitals capable of performing surgical interventions. Conservative treatments for cholesteatoma have been considered non-existent [2]. However, we have undertaken an evaluation to ascertain whether patients with limited cholesteatoma confined to the external auditory canal should be directed towards surgical intervention or be placed under conservative 'wait-and-see' surveillance. Moreover, a subset of patients who underwent tympanoplasty several decades ago found themselves devoid of further treatment options, enduring persistent accumulation of debris accompanied by recurrent cholesteatoma. Even in cases where complete removal of cholesteatoma within the external auditory canal was achieved, certain patients experienced recurrences [2].
The antimetabolite 5-fluorouracil (5-FU) has garnered significant utilization as a suppressive agent for countering the abnormal proliferation of carcinoma in diverse cancer chemotherapy settings [3,4]. Within the field of dermatology, 5-FU ointment has demonstrated efficacy in the elimination of primary lesions associated with actinic keratosis [5]. Following the seminal work by Smith et al., which indicated the potential of 5-FU in treating cholesteatoma, subsequent investigations have corroborated its capacity to curtail epithelial proliferation, thereby exhibiting promise against this condition [6,7]. Notably, experiments conducted on chinchillas, wherein cholesteatoma was induced through the application of chemical irritants, have further substantiated the effectiveness of 5-FU [8]. For patients who exhibit reluctance towards surgical interventions or decline such procedures, conservative management at ambulatory care facilities may emerge as a pertinent consideration [9]. In this context, topical 5-FU ointment therapy presents itself as a straightforward and non-invasive treatment option that holds significant potential in terms of patient-friendliness [10]. The direct resolution of cholesteatoma at the ambulatory care level can alleviate the burden experienced by affected individuals.
In this observational investigation, we explored conservative treatment strategies employed for cholesteatoma, with specific emphasis placed on the administration of 5-FU in the context of ambulatory care. The main objective of this study was to evaluate the practicality and effectiveness of 5-FU as a therapeutic agent in the ambulatory setting for cholesteatoma. Through the elucidation of potential advantages and discerning the outcomes associated with this treatment approach, our pursuit aimed to yield a valuable augmentation to the existing corpus of knowledge and propel the comprehension of conservative management choices for cholesteatoma to new heights.
Study Design
This case series study was conducted at the Department of Otolaryngology - Head and Neck Surgery in Nihon University Matsudo Hospital. It involved an in-depth evaluation of 15 ears from 14 patients who sought treatment for cholesteatoma between November 2007 and November 2020.
Patient Cohort
To ensure the selection of appropriate candidates for this investigation, specific criteria were employed. Patients included in this study displayed cholesteatoma localized within the external auditory canal, attic portion, or previously operated mastoid cavity. By focusing on individuals eligible for treatment at the ambulatory service center, this study maintains a practical approach. The utilization of computed tomography examinations played a crucial role in our evaluation of cholesteatoma extension and the accompanying bone erosion. Notably, prior to commencing any treatment, a thorough cleansing of the affected ears was conducted to ensure debris removal. Moreover, all extracted tissue underwent rigorous pathological examination, ensuring precise and accurate diagnoses.
Treatment Procedure
A targeted approach was employed to evaluate the efficacy of utilizing 5-FU cream as a treatment intervention. Precise application of approximately 0.3 mg of 5% 5-FU cream, sourced from Kyowa Kirin (Tokyo, Japan), was meticulously administered to the specific site of the cholesteatoma. This treatment regimen was carefully administered at least once a month, allowing for an adequate duration to evaluate the success of the intervention.
Evaluation of Treatment Outcomes
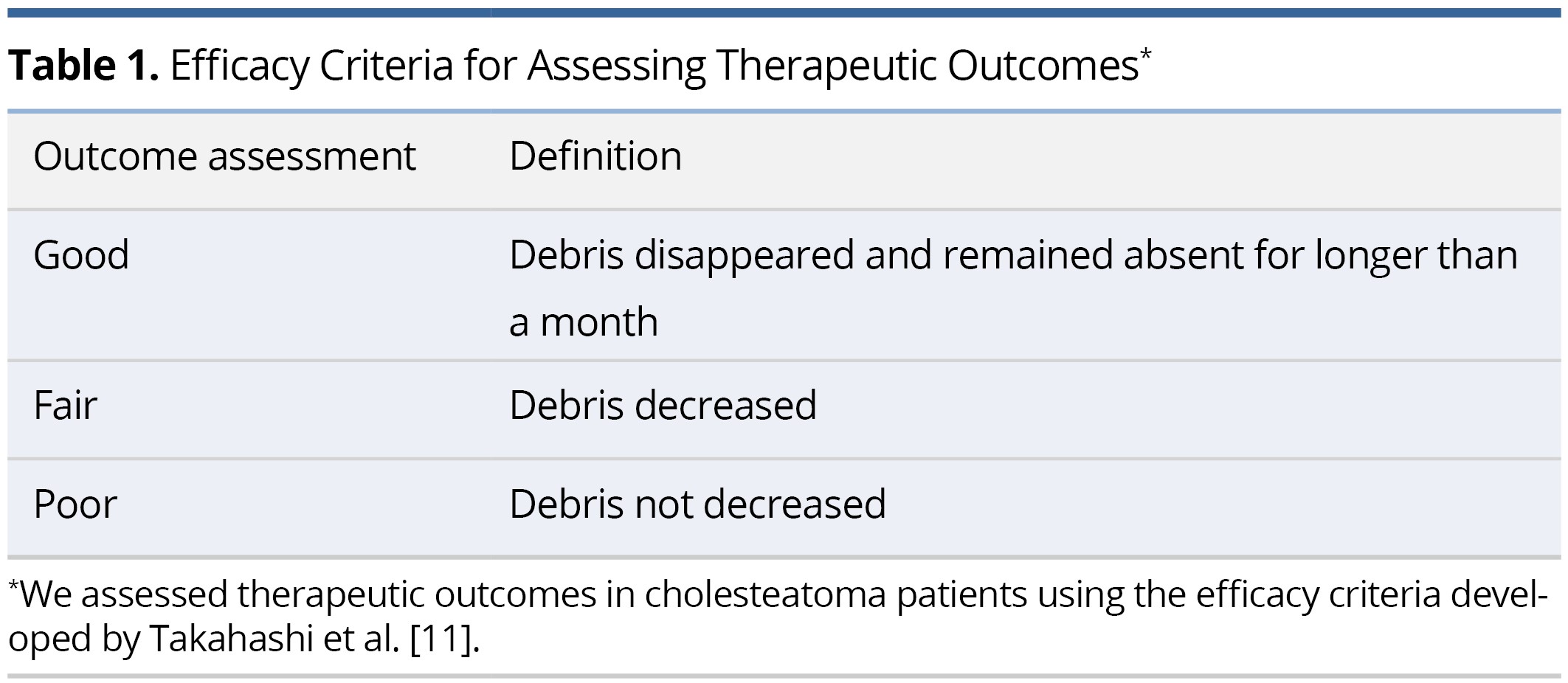
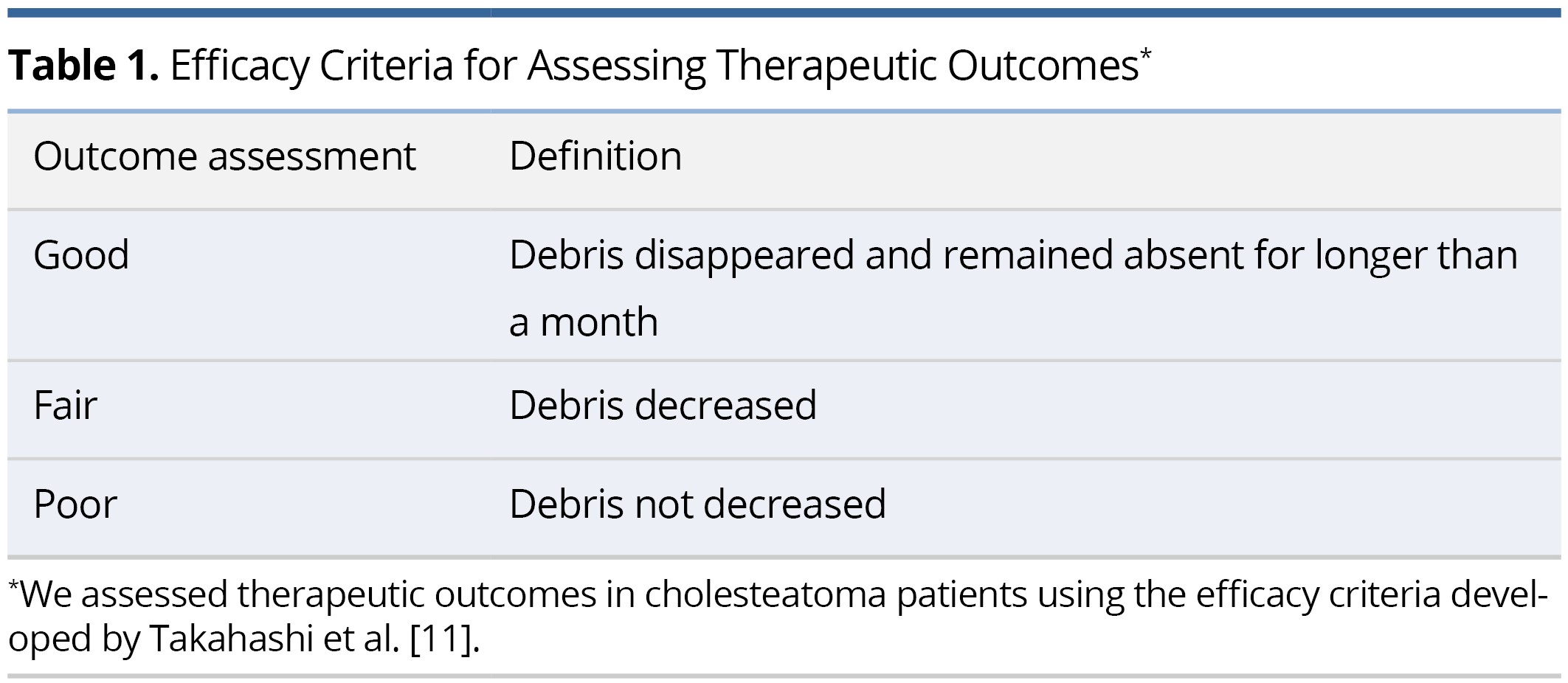
To assess the effectiveness of the treatment involving the application of 5-FU cream, we employed efficacy criteria developed by Takahashi et al. This method was specifically designed to evaluate therapeutic outcomes in patients diagnosed with cholesteatoma (Table 1) [11]. This approach allowed us to incorporate a more detailed framework to judge the intervention's success. A "Good" response signifies the total elimination of debris, which remains absent for over one month. A "Fair" response represents a decrease in debris, whereas a "Poor" response implies no observed reduction in debris.
Follow-up Procedures
For a thorough evaluation of treatment efficacy, we developed a rigorous follow-up protocol characterized by a close monitoring and comprehensive documentation of cholesteatoma progression on a monthly basis. This approach yielded invaluable insights into the intricate dynamics of growth and the enduring effects of the intervention. Additionally, for patients who exhibited no evidence of recurrence, we scheduled follow-up visits at regular intervals of 2-3 months, ensuring a minimum observation period of 6 months. By adhering to this rigorous methodology, we successfully portrayed the treatment's effectiveness.
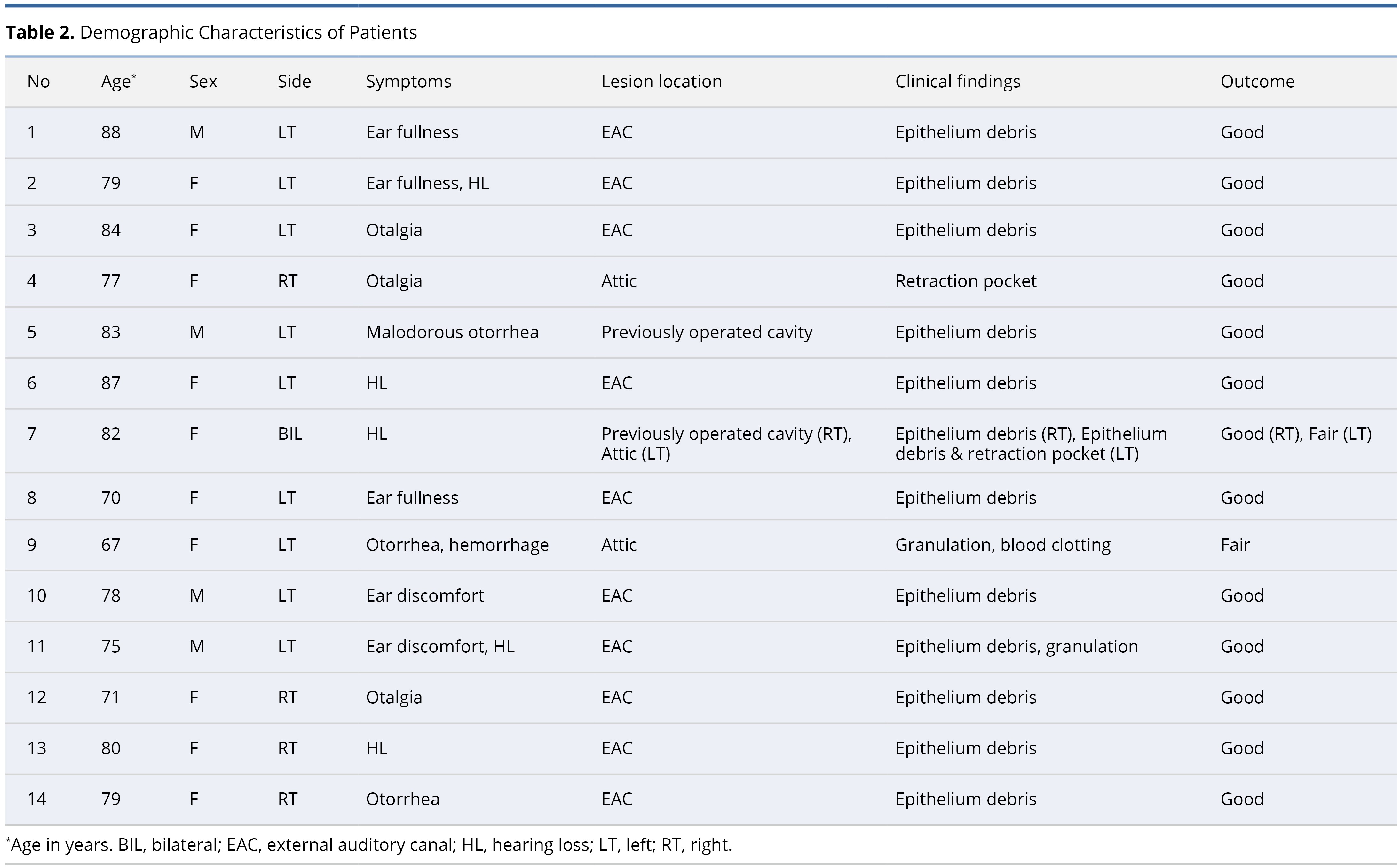
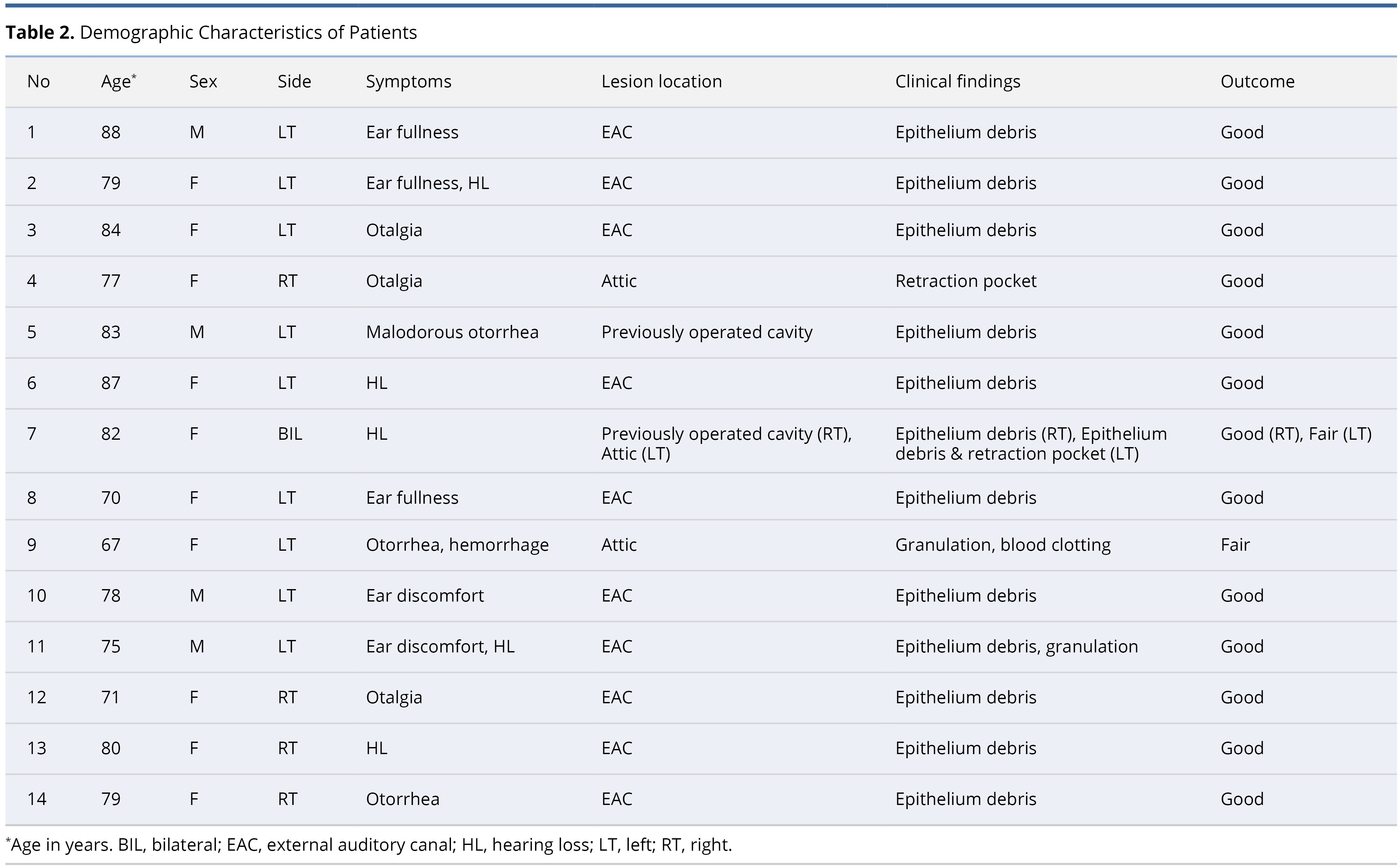
An extensive evaluation was undertaken on a group of 14 patients (15 ears) who sought medical assistance at our ambulatory healthcare facility, enabling us to obtain a comprehensive understanding of the demographic distribution and diverse range of symptoms associated with cholesteatoma. Table 2 provides a summary of the demographics, clinical manifestations, anatomical locations, and treatment outcomes observed within this specific patient group.
Demographic Distribution
The age range of the patients included in this study spanned from 67 to 88 years, with an average age of 78 years. This indicates an increased likelihood for the disease to manifest in later life. Notably, a significant majority of approximately 71% of the patients were female, suggesting a potential sex-biased predisposition in the prevalence of the disease within our cohort, warranting further investigation.
The left ear was predominantly affected by cholesteatoma, observed in nine patients, accounting for 60% of the cohort. In contrast, growth in the right ear was observed in only four patients (27%). Bilateral cholesteatoma, a rare occurrence, was identified in a single case, representing 13% of the population.
Symptomatology
The cohort of patients displayed a diverse array of clinical presentations related to cholesteatoma, illustrating substantial variability. Notably, hearing impairment was identified as the predominant symptom reported in 33% of patients. Additionally, otorrhea and otalgia were documented in 17% of patients each. Furthermore, aural fullness was observed in 13% of patients, while discomfort was experienced by 11% of individuals. Hemorrhage, being the least frequent symptom, manifested in 6% of patients.
Anatomical Location of Cholesteatoma
From an anatomical perspective, the cholesteatoma primarily manifested in the external auditory canal, affecting ten ears or 67% of the cases. A smaller number of occurrences were observed in the attic, accounting for three cases or 20%, while two cases or 13% were localized in the previously operated mastoid cavities.
Clinical and Pathological Correlations
According to the clinical diagnosis, a significant proportion of 87% of ears demonstrated the presence of epithelial debris, which is characteristic of cholesteatoma. However, in two ears, a more intricate situation was observed, with the coexistence of granulation alongside epithelial debris, accounting for 13% of the cases. A meticulous pathological examination confirmed the clinical diagnosis, with all specimens unequivocally aligned with cholesteatoma characteristics.
Assessment of Treatment Outcomes
Despite the severity of the cholesteatoma condition, none of the patients opted for surgical intervention. Instead, they underwent a treatment regimen involving 5-FU. The overall prognosis of the patients was encouraging, with 13 out of 15 ears exhibiting a "Good" outcome, characterized by the absence of cholesteatoma debris for a duration exceeding one month [11]. The remaining two ears were classified as "Fair" due to the absence of recurrence following repeated 5-FU therapy over a period of two months. Importantly, none of the ears were categorized as having "poor" treatment outcomes.
Case Reports
We hereby present two illuminating case studies that exemplify the triumphant eradication of cholesteatomas, thus emphasizing the therapeutic efficacy of 5-FU cream within clinical settings.
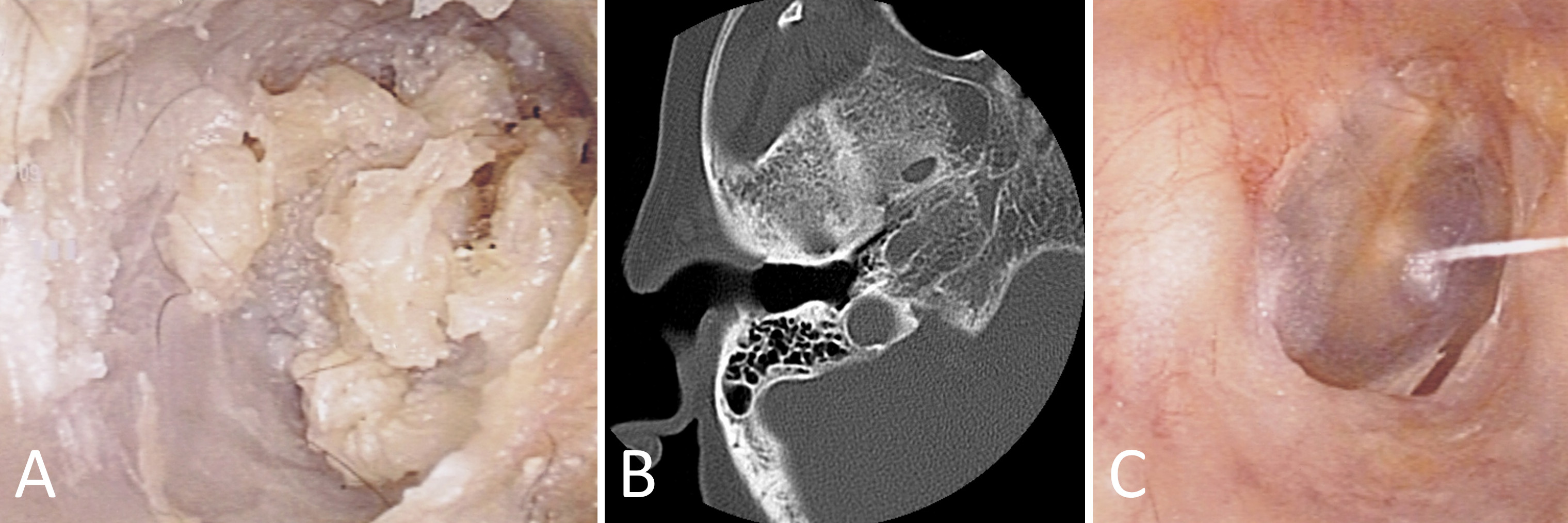
Our first case revolves around a 72-year-old female patient who reported persistent right otalgia. Initial clinical assessment disclosed the presence of tissue debris within the ear canal (Figure 1A). To comprehensively evaluate the extent of the pathology, computed tomography imaging was employed, which revealed an absence of bone erosion in the external auditory canal or mastoid bone. Furthermore, intact tympanic membrane and middle ear structures confirmed the diagnosis of external auditory canal cholesteatoma (Figure 1B). The collected specimens underwent meticulous pathological examination in accordance with the distinctive features of cholesteatomas. Subsequently, the patient underwent treatment involving the application of topical 5-FU cream. Remarkably, this therapeutic regimen led to the complete eradication of the cholesteatoma, with no recurrence observed during the follow-up period (Figure 1C).
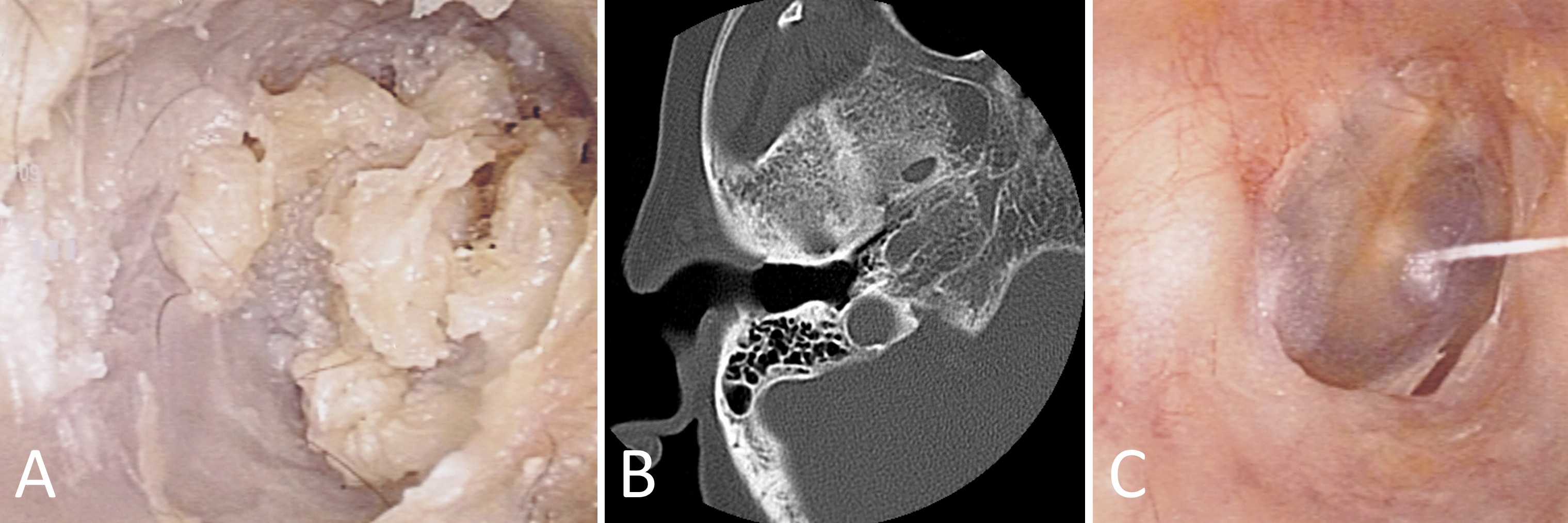
Figure 1. This case report showcases the efficacious management of external auditory canal cholesteatoma in a 72-year-old female patient with persistent right otalgia. (A) Initial clinical assessment reveals the presence of tissue debris within the ear canal, indicating the potential pathology. (B) Computed tomography imaging portrays a reassuring absence of bone erosion in both the external auditory canal and mastoid bone. Moreover, the intact tympanic membrane and middle ear structures provide definitive confirmation of external auditory canal cholesteatoma. (C) Through the meticulous application of topical 5-FU cream, the cholesteatoma is completely eradicated, resulting in a remarkable absence of recurrence during the follow-up period.
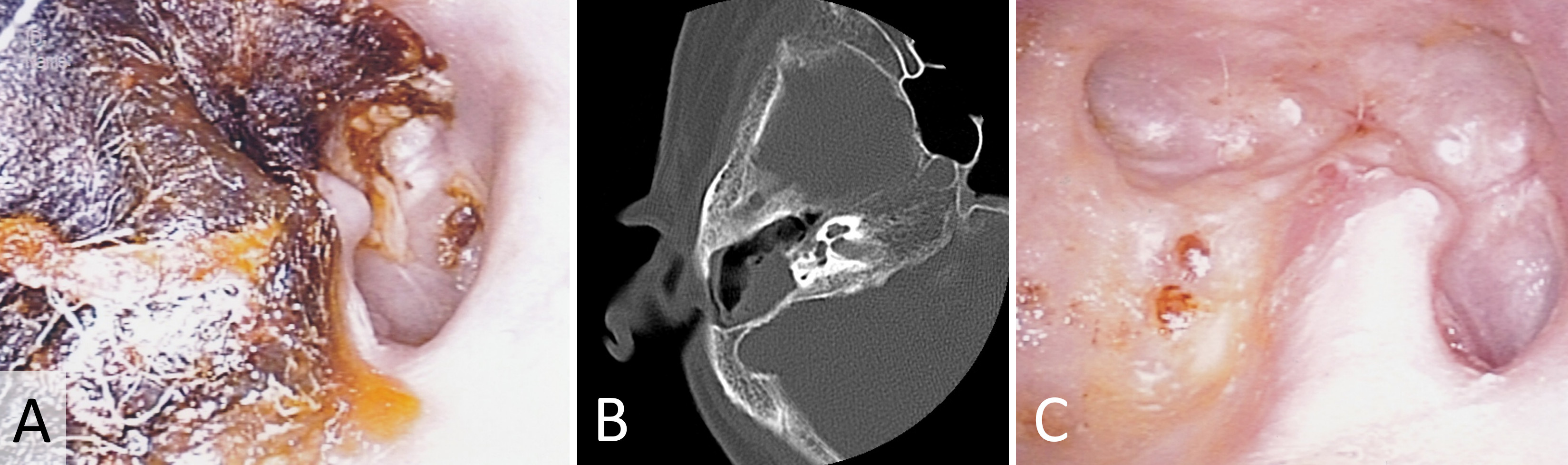
Shifting focus to our second case, an 82-year-old female patient presented with bilateral hearing loss. Examination of the previously operated area of the right ear revealed the presence of tissue debris (Figure 2A). Subsequent computed tomography scanning unveiled a soft tissue image within the previously operated mastoid cavity, indicative of cholesteatoma (Figure 2B). The gathered specimens underwent a rigorous pathological examination that adhered closely to the distinct characteristics associated with cholesteatomas. In response, prompt treatment was initiated utilizing topical 5-FU cream, effectively eliminating the cholesteatoma without any recurrence detected (Figure 2C).
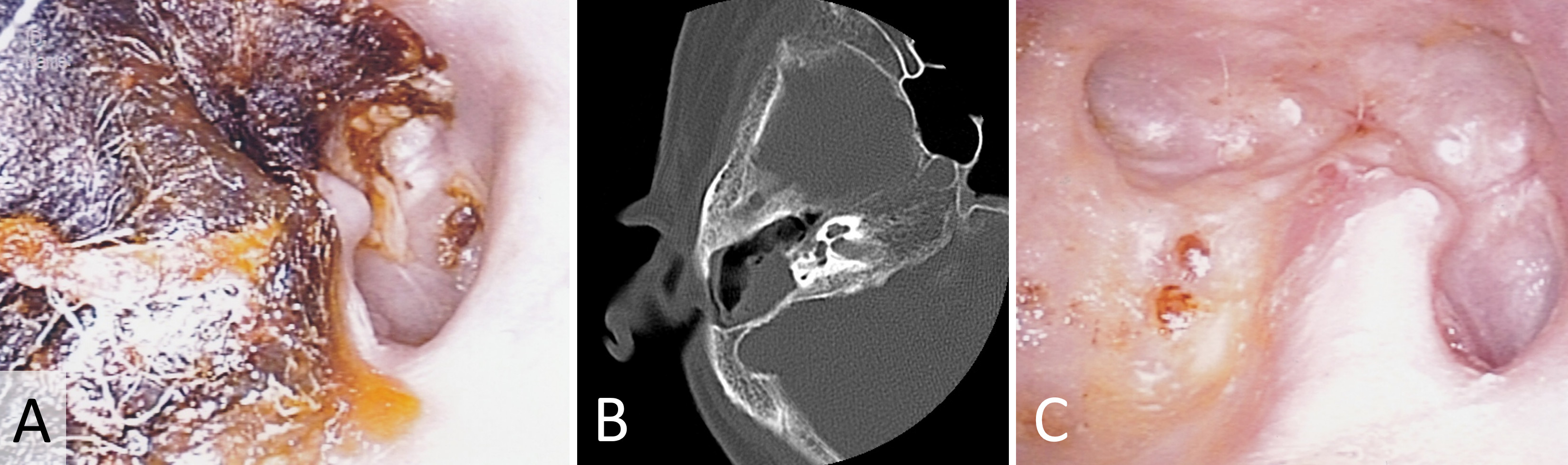
Figure 2. In this case study, we present the successful eradication of cholesteatoma in an 82-year-old female patient who initially presented with bilateral hearing loss. (A) Upon examination of the previously operated area in the right ear, the presence of tissue debris is observed, suggesting the possibility of cholesteatoma. (B) Subsequent computed tomography scanning reveals the presence of a soft tissue image within the previously operated mastoid cavity, confirming the diagnosis of cholesteatoma. (C) By employing a treatment approach involving the application of topical 5-FU cream, the cholesteatoma is effectively eliminated without any detected recurrence.
These two remarkable cases provide compelling and indisputable evidence, firmly establishing the remarkable potential of topical 5-FU cream as an efficacious and viable treatment modality for external auditory canal cholesteatomas. The resounding success observed in both patients not only highlights the feasibility of this non-surgical approach in achieving complete cholesteatoma removal but also effectively mitigates the risk of recurrence. Moreover, the utilization of 5-FU cream presents a promising and enticing alternative to invasive surgical procedures, effectively minimizing the inherent risks and complications associated with such interventions.
Cholesteatoma, a progressive and potentially devastating ear condition characterized by the abnormal growth of keratinized squamous epithelium, has traditionally been managed through surgical intervention. However, recent pioneering efforts have focused on conservative management strategies as a viable alternative, aiming to minimize the invasiveness of treatment while ensuring favorable clinical outcomes. In this context, our institution has embarked on an innovative approach to cholesteatoma treatment, utilizing the antimetabolite 5-FU within an ambulatory care setting, and subsequently evaluating its efficacy and safety.
Our approach to cholesteatoma treatment utilizing 5-FU within an ambulatory care setting demonstrated promising clinical outcomes. By adopting conservative management strategies, we were able to address cholesteatoma primarily localized within the external auditory canal, offering a less invasive alternative to surgical intervention. The efficacy and safety of 5-FU topical cream, as evidenced by the disappearance of cholesteatoma debris in most cases without observed systemic adverse effects, support its utilization in ambulatory care settings. Although our study has inherent limitations, it offers valuable insights into the potential of non-surgical strategies, specifically the utilization of 5-FU, in the management of cholesteatoma.
Contrary to the findings reported by Naim et al. [12], our study revealed that hearing loss emerged as the predominant symptom among the patients in our cohort. Remarkably, many of these individuals exhibited a profusion of epithelial debris closely resembling cerumen within the external auditory canal. Given the advanced age of most of our patients, further investigation is warranted to discern whether this hearing loss is functionally related or directly associated with cholesteatoma.
In our cohort, cholesteatoma primarily localized within the external auditory canal, although we successfully accessed the attic and previously operated mastoid cavities in more advanced cases. Conventionally, surgical intervention has been favored for managing cholesteatoma, especially when extensive bone exposure leads to keratin debris accumulation, periostitis, and erosion of the epithelial tissue [13]. However, in our study, we aimed to explore the potential of ambulatory care management, which offers a less invasive approach to treatment.
Instrumental in our assessment of cholesteatoma extension and associated bone erosion was the use of computed tomography examinations [14]. These imaging techniques aided in identifying cases where cholesteatoma extended into the middle ear, while most of our patient cohort demonstrated localized involvement within the external auditory canal.
The selection of 5-FU as a therapeutic intervention in our study was based on its antimetabolite characteristics. Through its ability to impede DNA synthesis, 5-FU effectively halts cellular growth and triggers cell death in neoplastic cells [3,15]. Beyond its well-documented efficacy against neoplasms, 5-FU has exhibited promising results in the treatment of cholesteatoma [16]. These findings provide further support for the utilization of 5-FU within ambulatory care settings for cholesteatoma management.
To assess the effectiveness of the treatment involving the application of 5-FU cream, we utilized Takahashi's efficacy criteria [11]. This approach allowed us to incorporate a more detailed framework to judge the intervention's success. Following the treatment regimen involving 5-FU, the overall prognosis yielded positive results, with 87% of the ears categorized as 'good' and 13% as 'fair,' thus providing validation for the potential of non-surgical approaches to managing cholesteatoma. Notably, our study demonstrated that all cholesteatoma cases responded effectively to topical 5-FU cream treatment, with no observed systemic adverse effects, thus corroborating the findings of Smith et al. [6].
Compared to conventional anticancer drugs, topical treatments generally present milder systemic side effects. Reports from dermatological applications of 5-FU for malignant tumors have indicated that erythema and erosion are the most commonly reported adverse effects [16]. Recently, the combination of 5-FU with sodium 2-mercaptoethanesulfonate (MESNA), a synthetic sulfur compound that disrupts epithelial disulfide bonds, has shown promise in rat studies for cholesteatoma treatment [17]. Interestingly, MESNA has already been utilized as an adjunct in cholesteatoma surgery [18], thereby suggesting that combined therapy with 5-FU and MESNA may hold potential as a future ambulatory care option.
Study Limitations
While our study offers valuable insights into the potential of non-surgical approaches, such as 5-FU, for cholesteatoma management, it is essential to acknowledge certain limitations in our research. Firstly, the absence of a control group prevents us from directly comparing the outcomes of conservative management with those of surgical intervention. Future studies could incorporate a randomized controlled design to address this limitation and provide more robust evidence regarding the efficacy of 5-FU in an ambulatory care setting.
Furthermore, it is important to note that our study population was limited to a single ambulatory service center, which may introduce biases and limit the generalizability of our findings. To overcome this limitation, multi-center studies involving diverse populations would be beneficial in assessing the broader applicability of our approach. By including different demographic groups and varying levels of disease severity, we can gain a more comprehensive understanding of the effectiveness of 5-FU in managing cholesteatoma across different patient profiles.
Additionally, the advanced age of our study cohort raises questions about the generalizability of our findings to younger patients. Cholesteatoma can affect individuals of all ages, and it is crucial to investigate the effectiveness and safety of conservative management strategies, such as 5-FU, in younger populations. Future research endeavors should aim to include a broader age range to address this important aspect and guide treatment decisions across different age groups.
Despite these limitations, our study sheds light on the potential of non-surgical approaches for cholesteatoma management, specifically using 5-FU in an ambulatory care setting. The favorable outcomes observed in our cohort, coupled with the absence of systemic adverse effects, highlight the promise of this therapeutic strategy. Moving forward, further investigation is warranted to optimize treatment protocols, explore potential combination therapies, and establish standardized guidelines for the use of 5-FU in cholesteatoma management.
Research highlights that topical 5-FU cream may provide significant advantages for susceptible demographics, such as the elderly and those in remote regions with constrained access to specialized healthcare. The findings indicate that this treatment method could serve as an affordable, accessible option, potentially alleviating strain on healthcare resources. Nevertheless, additional studies are required to confirm the lasting safety and effectiveness of 5-FU cream in cholesteatoma management and to identify the ideal parameters for patient selection.
Received date: March 13, 2023
Accepted date: April 25, 2023
Published date: May 23, 2023
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills in order to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2023 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
The present study demonstrated that TEES could be a satisfying alternative to traditional microscopic surgery for the management of congenital cholesteatoma, even in pediatric patients. However, one-handed surgery demands greater skill and requires more practice to achieve a good outcome.
The authors describe a 41-year-old man who suffered retraction-related complications that may have been missed or delayed. The present case illustrates the potential dangers associated with tympanic retraction pockets, despite the fact that their bottoms are clear and clean. The article discusses the reasons for the lack of consensus among otologists regarding the appropriate way to treat tympanic membrane retractions. There is further discussion regarding the challenges associated with early surgical intervention.
The authors demonstrate their commitment to their research and expertise by thoroughly addressing initial concerns. They effectively elucidate every aspect of the issue in a detailed manner, leading to a more convincing and extensive exploration of the research topic. The manuscript has undergone significant enhancement, reflecting the authors' scrupulousness and precision. I believe that the revised manuscript will pique the curiosity of researchers and scholars and make a substantial contribution to the field. It is praiseworthy that the authors managed to integrate feedback and refine the manuscript's organization and substance. Considering this, I endorse the publication of the revised manuscript in its current form.
ResponseI would like to extend my deepest gratitude towards you. Your comments have been enlightening and they indeed fuel our motivation for future research endeavors.
In the revised manuscript, the authors exhibit an outstanding degree of dedication and proficiency, addressing initial concerns effectively. Their exhaustive and detailed explanations resolve previously raised issues and lead to a more in-depth examination of the research topic. This revised version is poised to capture the attention of researchers and scholars, thus making a significant contribution to the field. Given the exceptional research prowess demonstrated in the revised manuscript, it warrants publication in the form in which it stands. I would like to make one last recommendation regarding the citation of References 12 and 13 within the Discussion section. In this section, the authors state, "In our patient population, most ears were localized in the EAC upon CT examination. Two ears of postoperative cholesteatoma exhibited a deficit of EAC involving the cells in the mastoid bone [12]. However, the CT feature of cholesteatoma in the EAC is depicted only as soft tissue mass, which cannot distinguish cholesteatoma from granulation [13]." The inclusion of references 12 and 13 in this section appears unrelated to the study's findings, as they are connected to different studies conducted by separate teams. As the primary purpose of this section is to report the study's outcomes, it would be beneficial for the authors to provide further clarification regarding their decision to reference these sources. Doing so will aid readers in understanding the rationale behind their inclusion and reduce any potential confusion or misinterpretation of the study's results.
ResponseI want to convey my deepest thanks for your perceptive feedback. Your comments have been instrumental in guiding our journey and they indeed provide a substantial impetus for our upcoming research work. Adhering to your noteworthy advice, we have excised sections that could potentially cause misunderstanding among the readers.
Niwa H, Utsunomiya T, Kuyama Y, Makiyama Y. A novel strategy for conservative management of external auditory canal cholesteatoma: Employing 5-fluorouracil in ambulatory care for select patients. Arch Otorhinolaryngol Head Neck Surg 2023;7(1):4. https://doi.org/10.24983/scitemed.aohns.2022.00172