Epistaxis (i.e., nosebleed) is a common otolaryngologic emergency; however, it is seldom life-threatening and most minor nosebleeds stop on their own or under primary care from medical staff. Nonetheless, cases of recurrent epistaxis should be checked by an otolaryngologist, and severe nosebleeds should be referred to the emergency department to avoid adverse consequences, including hypovolemic shock or death. This paper reviews current advances in our understanding of epistaxis as well as updated treatment algorithms to assist clinicians in optimizing outcomes.
Epistaxis refers to hemorrhaging from the nose (i.e., nosebleed). This condition is among the most common otolaryngologic emergency, affecting roughly 60% of individuals [1], of whom 6-10% require medical attention [2-6]. Most individuals experiencing epistaxis do not pursue medical help; however, if not dealt with in an appropriate manner, cases of extreme bleeding can lead to airway obstruction, aspiration, asphyxiation, hypovolemic shock, or even death [7-9]. Recent advances in endoscopy have facilitated nasal examinations; however, the effective management of this condition depends on an awareness of the probable sources of epistaxis and a comprehensive understanding of the nasal structure. This paper presents a review of the literature on epistaxis including recent advances in its treatment. This article also provides stepwise algorithms to guide clinicians through the decision process underlying the management of epistaxis.
Epistaxis occurs in up to 60% of the general population [6]. The incidence of epistaxis presents a bimodal distribution, with most cases occurring in individuals below the age of 10 and above the age of 70 [6,10-12]. The average age of patients hospitalized for nosebleed is 70 years old [13]. Elderly individuals account for 40% of the cases requiring medical intervention, due primarily to the fact that bleeding events among the elderly can have severe consequences. Children tend to present with uncomplicated nosebleeds in the anterior region, which seldom require surgical intervention [14]. Despite the low prevalence of epistaxis among children below the age of 2, all such cases should be carefully examined for signs of trauma, the presence of an external object, or systemic illness [15]. Males are slightly more likely than are females to experience epistaxis [12,16], due perhaps to the protective effects of estrogen [11,17-19]. Thus, there is a predominance of males among hospitalized patients below the age of 49 and a more equal sex distribution above this point.
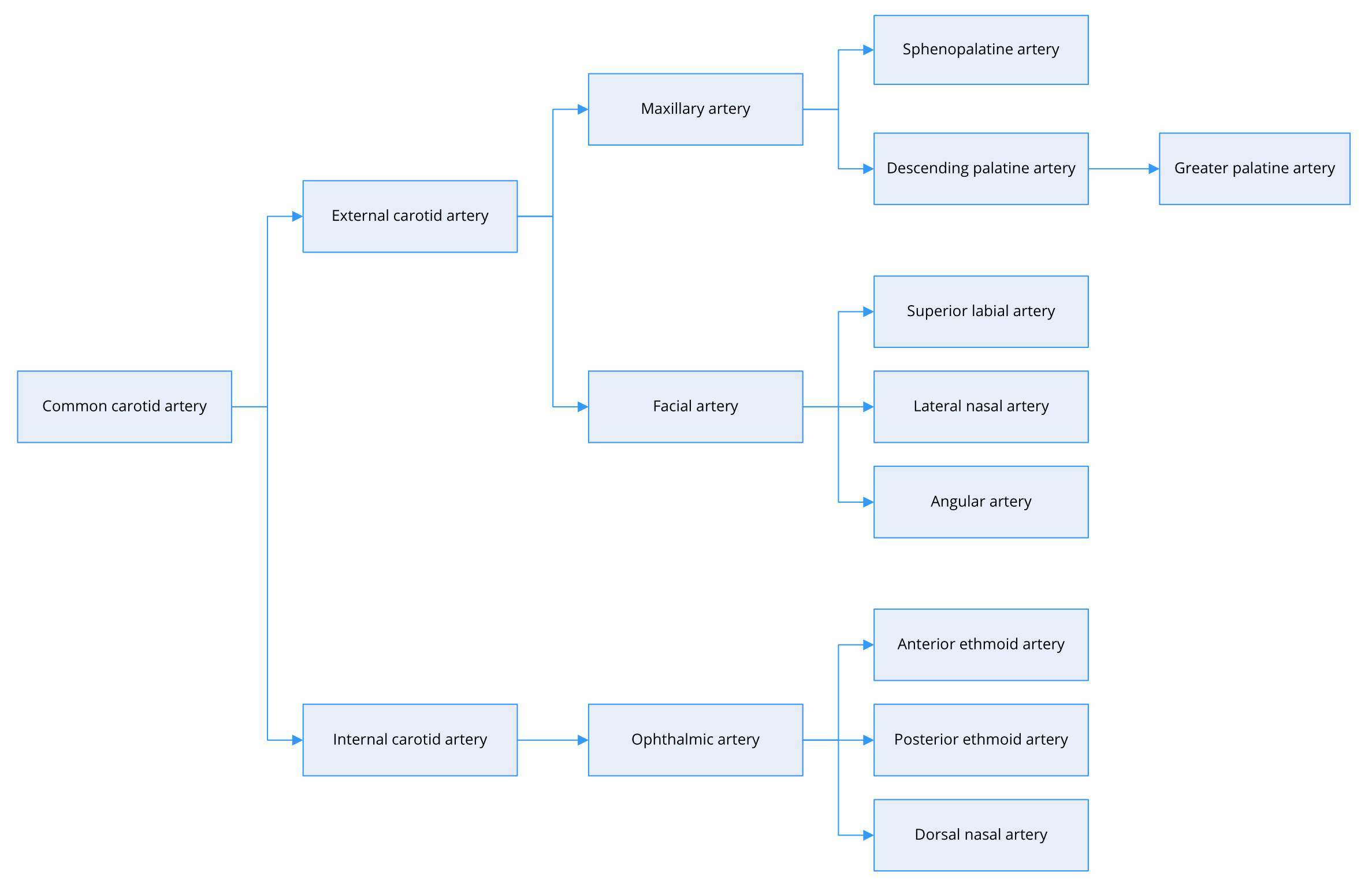
The primary functions of the nose include warming and humidifying inhaled air. This requires copious quantities of blood from external and internal carotid arteries (Figure 1). The external carotid artery serves as the major contributor and provides arterial flow primarily via the maxillary artery and secondarily via the facial artery. The maxillary artery splits into several branches, including the sphenopalatine artery and descending palatine artery. The sphenopalatine artery supplies the mucosa in most of the nasal septum and turbinates. The descending palatine artery has two or three branches, including the greater palatine artery, which takes a circuitous course passing inferiorly through the greater palatine canal and foramen to supply the nasal septum and floor of the nose. The facial artery divides into various branches, including the angular artery, lateral nasal artery, and superior labial artery, which supplies the septum and nasal alae [7]. The internal carotid artery supplies the region above the middle turbinate broadly via the anterior and posterior ethmoid arteries.
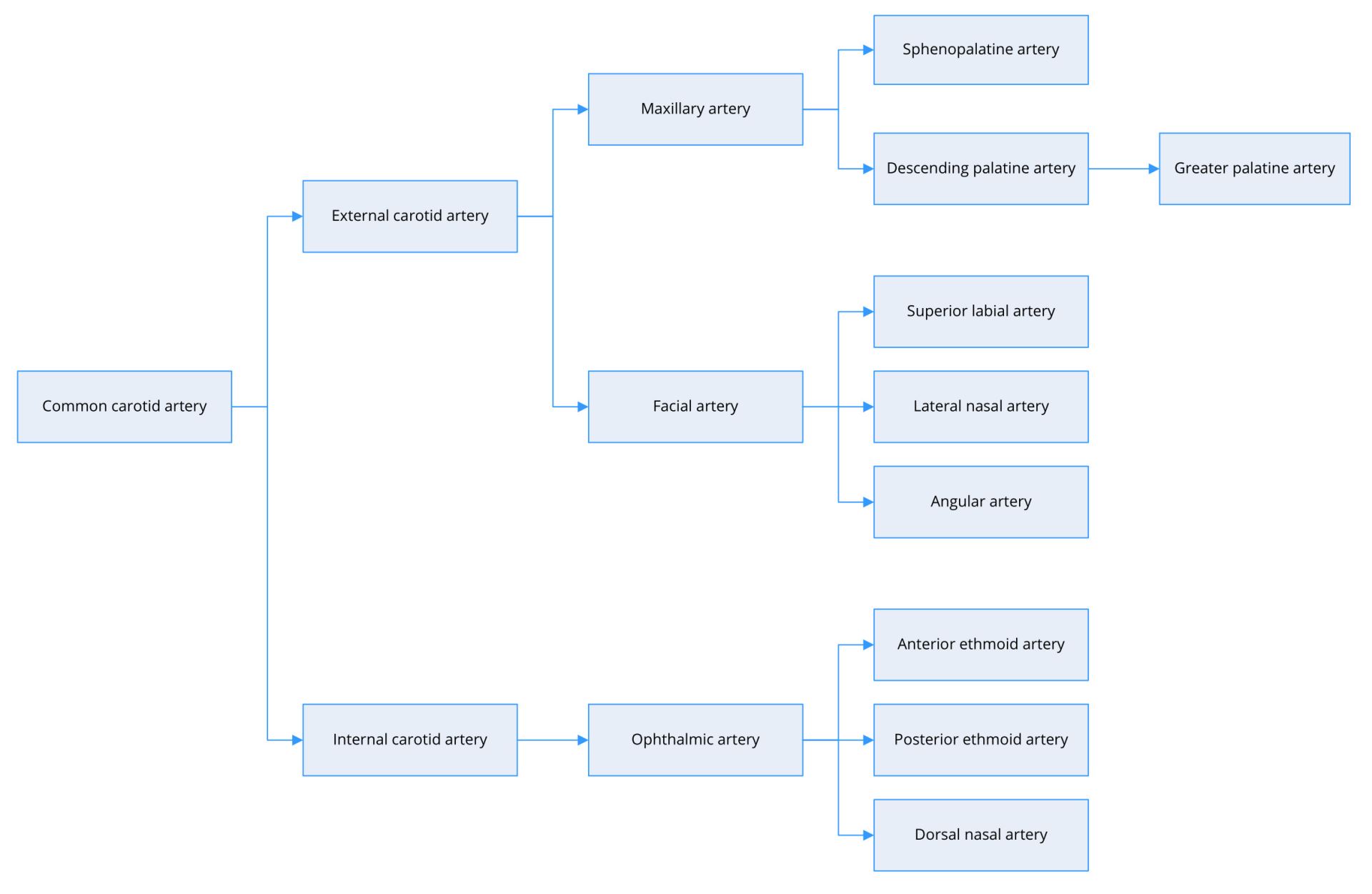
Figure 1. Arterial supply of the nasal cavity.
Nosebleeds are categorized as primary or secondary. Primary nosebleeds are idiopathic and spontaneous, whereas secondary events have definite causes, such as trauma or the use of anticoagulants [20]. Despite the complexity of the circulatory system in the nose, on the other hand, nosebleeds can also be categorized simply as anterior or posterior, depending upon the source of bleeding. Most cases of epistaxis (90% to 95%) occur at an anastomosis referred to as Kiesselbach’s plexus or Little’s area in the inferior region of the anterior septum [11,21-25]. This area is saturated with branches of the superior labial artery, anterior and posterior ethmoid arteries, sphenopalatine artery, and greater palatine artery. These arteries are supplied by base branches of the internal and external carotid arteries [26]. A relatively small number of cases of epistaxis (5 to 10% of the total) are associated with arteries beneath the posterior end of the inferior turbinate, which forms Woodruff’s plexus [21,24,27].
Epistaxis is a multifactorial entity. The etiology of nosebleeds can be categorized according to the cause as local, systemic, environmental, or drug-related. The local origins of epistaxis include trauma, intranasal neoplasia, inflammatory disease, and septal abnormality. A rich vascular network renders the nose susceptible to epistaxis. This is exacerbated by the fact that blood vessels within the nasal mucosa are outwardly situated, leaving them exposed to damage. Most of the cases encountered by clinicians involve some sort of trauma or irritation to the mucosa and/or associated blood vessels [28,29]. One common example is injury resulting from the insertion of a finger (nose picking) [30]. Kiesselbach’s plexus is completely exposed just within the cavity of the nose. Nosebleeds due to blunt trauma are typically from an anterior source. The introduction of a foreign object into the nose can cause profuse bleeding. In cases where the item has remained in that position for more than 24 hours, bleeding may be accompanied by pus-filled nasal discharge [31]. Nasotracheal intubation, nasogastric tube insertion, and the chronic use of nasal cannula are some of the most common causes of epistaxis among hospitalized patients. Fiberoptic guidance during nasotracheal intubation has been shown to reduce the incidence and severity of epistaxis, compared with conventional (unguided) insertion [32]. Recurrent epistaxis can be caused by sinonasal tumors, including squamous cell carcinoma, adenoid cystic carcinoma, melanoma, inverted papilloma, or other rare tumors [25,33]. It is notable that nasopharyngeal cancers are far more common among southeast Asians than among Caucasians [34]. The possibility of nasopharyngeal cancer needs to be excluded in southeast Asians with nosebleeds, in particular if specific symptoms and signs are concurrently observed, such as unilateral aural fullness and neck masses. When dealing with teenagers suffering from nosebleeds, it is imperative to consider juvenile nasopharyngeal angiofibroma, a benign but aggressive and expansile tumor that can invade adjacent structures resulting in extensive bleeding [35]. Cases of neoplasia are rare; however, it is important that clinicians conduct a rigorous examination to conclusively exclude this possibility.
Systemic causes of epistaxis include hypertension, cirrhosis, alcoholism, aberrations in clotting ability, inherited bleeding diatheses, and vascular/cardiovascular diseases [20,36,37]. Despite the fact that hypertension is not a direct cause of epistaxis, it has been linked to cases of severe or refractory epistaxis [15,36,38]. Some researchers have surmised that hypertension associated with an underlying vasculopathy that includes atherosclerosis may be a risk factor for epistaxis [39]; however, there is little evidence to support this assertion [40]. Furthermore, elevated arterial blood pressure at the onset of epistaxis may also be associated with stress and/or white coat syndrome [41]. Alcohol has been linked to an elevated risk of epistaxis [42]. Alcohol consumption reduces platelet aggregation, which can prolong the duration of bleeding. Hemodynamic changes may also be associated with some cases of epistaxis. Epistaxis is the most common manifestation in patients with hereditary hemorrhagic telangiectasia (HHT, also called Osler-Weber-Rendu syndrome), occurring in 90% to 95% of patients [43]. HHT is an autosomal dominant vascular disorder, which has been somewhat under reported [44]. Several patients with HHT have been found to be resistant to treatment for epistaxis, including oral estrogen, topical estriol plus argon plasma coagulation, oral tamoxifen, oral tranexamic acid, submucosal bevacizumab, topical bevacizumab, and sclerotherapy [43,45-50]. Intravenous bevacizumab [51] and thalidomide [52,53] have been reported as effective and safe in reducing the incidence of epistaxis in HHT patients; however, further research is required to validate the benefits in terms of quality of life. Many patients with other bleeding ailments suffer from recurrent episodes of epistaxis [45]. It is essential to consider a bleeding diathesis when treating patients with recurrent spontaneous epistaxis [11,54].
Environmental factors also play an important role in the onset of epistaxis. There is a general increase in epistaxis in the winter months, due to lower temperatures and drier air [11,16,36,55]. Dry air tends to irritate the mucosa, leaving it susceptible to bleeding under even slight aggravation. Irritation from nasal infection or allergic rhinitis can also make the nasal mucosa friable, following the inflammation of nasal turbinates [13,42,56-61]. Epistaxis has been associated with the topical use of nasal steroids; however, the incidence of epistaxis among patients taking these drugs is only slightly above the incidence of those taking a placebo, and the symptoms are usually minor and self-limiting [62-66]. Manfredini et al. linked the incidence of epistaxis to cardiac rhythms. They found that the time of epistaxis occurrence presents a biphasic circadian pattern, with a primary peak in the morning, a smaller secondary peak in the evening, and a nocturnal nadir [67]. The authors commented that this biphasic pattern closely resembles the circadian rhythm of blood pressure, suggesting that blood pressure may be associated with epistaxis.
A number of drugs such as warfarin, dipyridamole, rivaroxaban, and nonsteroidal anti-inflammatory drugs (NSAIDs) can affect blood coagulation [1,29,40,68]. NSAIDs, including aspirin and ibuprofen, are the most common drugs that may interfere with coagulation [45]; however, researchers have yet to establish a definitive causal association between the use of NSAIDs and epistaxis [69-71]. It has been estimated that 24% to 33% of patients admitted for nosebleeds are taking anticoagulants or antiplatelet medications [2]. Vitamin K antagonists, such as phenprocoumon, have also been shown to contribute to recurrent epistaxis [68,72]. It has been reported that specific serotonin reuptake inhibitors and antibiotics can induce epistaxis; however, most of those bleeding episodes are mild and easily reversed [1]. Overall, it is important that clinicians refer to the medication history of patients with epistaxis and consider alternative causes.
Patients undergoing treatment with anticoagulants face an elevated risk of nosebleeds; however, there are as yet no clear guidelines regarding the means by which epistaxis patients should be treated in cases of an elevated international normalized ratio (INR) of 5 or more [11]. There is also a degree of controversy regarding whether patients with thromboembolic risks presenting with a minor nosebleed or bleeding from an inaccessible site should be treated using local measures or surgical interventions. Further high-quality research will be required to resolve this issue [73,74]. At present, clinicians base their selection of treatment methods on the site and extent of bleeding, history of bleeding, the perceived likelihood of progression to more severe bleeding, comorbidities including hypertension and renal insufficiency, INR level, and the likelihood that INR has been supratherapeutic over the previous few measurements [75]. Treatment options include ceasing warfarin treatment, ceasing warfarin with vitamin K, or initiating aggressive anticoagulation reversal for patients presenting with pronounced bleeding [73]. As long as hemostasis can be achieved, patients with mild nasal bleeding can safely continue with their warfarin regimen, albeit with suitable adjustments [28].
Evaluation of Epistaxis
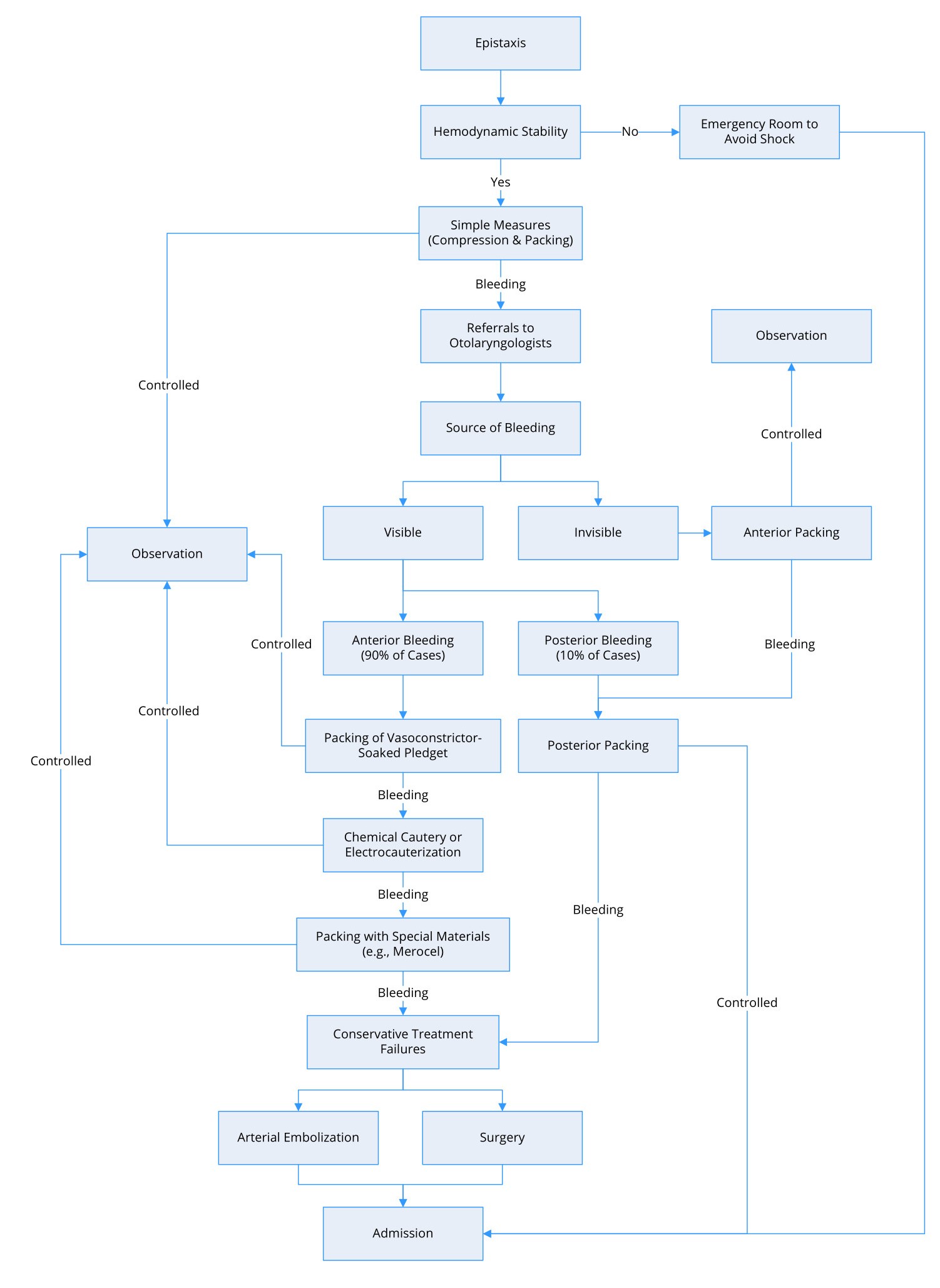
Figure 2 presents a flow diagram detailing the evaluation and management of patients with epistaxis. Before examining patients with epistaxis, practitioners must ensure that the patient has a patent airway and cardiovascular stability. In cases of extensive bleeding and/or low blood pressure, it is important to transport the patients to the emergency department as soon as possible to avoid subsequent consequences. Clinicians in the emergency department should collect data related to blood type and cross-matching for possible blood transfusions [76,77].
After confirming hemodynamic stability, clinicians should collect a focused history to identify the factors that could contribute to epistaxis. Clinicians should obtain an account of the acute episodes and previous incidents (if any), including the extent, seriousness, frequency, and laterality of nosebleeds, as well as the methods used to control them. In cases of severe hemorrhaging or refractory epistaxis, it is important to consider conditions that predispose the patient to bleeding or other related injuries, including coagulation disorders, medications, and alcohol consumption. It is also crucial that clinicians inquire about hematemesis and the occurrence of black, tarry stools [45]. Patients undergoing treatment with anticoagulants should be evaluated to identify potential hemostatic disorders. Routine clotting screening is not required for patients who do not present with relevant risk factors [78,79].
The initial evaluation is meant to eliminate factors that could predispose the patient to epistaxis and identify the source of bleeding. Epistaxis can be classified as anterior or posterior according to the source of bleeding, and it is crucial to differentiate between the two. Anterior nosebleeds are the most common and are usually self-limited. Posterior epistaxis generally involves more profuse bleeding and cannot always be managed in a primary care setting [11]. Slight bleeding is generally indicative of an anterior source; however, a large volume of blood does not necessarily indicate a posterior source. Bleeding from both the nostrils is a clear indication of a posterior source. Bilateral epistaxis can occur in patients with a septal defect or bilateral nasal lesions.
Initial Management of Epistaxis
Initial management includes the application of pressure to areas of bleeding using cotton or gauze saturated in a topical decongestant [80,81]. In cases where a clinician is unable to determine the source of the bleeding, nasal packing should be applied in both nasal cavities using vasoconstrictor-soaked pledgets to hasten hemostasis. Vasoconstrictors, such as oxymetazoline, phenylephrine, or lidocaine, can reduce topical bleeding through vasoconstriction without the risk of elevating systemic blood pressure [80,82]. If this proves insufficient to halt bleeding, then the source is most likely posterior, and posterior packing may be required. Tilting the head forward helps to prevent the pooling of blood in the posterior pharynx and thereby may prevent nausea and airway obstruction.
The severity of epistaxis can range from light nosebleeds to potentially fatal bleeding. Minor events are easily managed in a clinical environment; however, major events often require hospital admission and even surgical intervention. In the event that the patient does not respond to initial treatment, efforts must be made to find the cause of the bleeding [6,68,83]. Children must be examined closely for a foreign body or nasal mass to ensure that epistaxis is benign [84]. Bleeding disorders should also be clinically excluded for patients with recurrent epistaxis.
Treating Anterior Epistaxis
Compression is the primary treatment mode for minor anterior epistaxis. This involves having the patient bend forward at the waist while sitting up to avoid swallowing blood. The clinician then applies pressure by grasping the alae distally and pinching them tightly against the septum without releasing the pressure for 5 to 10 minutes. If the initial treatment fails to stop the bleeding, a plug of cotton wool or a pledget can be inserted into the nasal cavity. Blood clots accumulated in the pharynx should be gently removed by suction, and a cold compress can be applied to the bridge of the nose before referring the patient to a specialist.
Patients lacking hemodynamic stability should be immediately referred to the emergency room for stabilization. Stadler et al. reported that an emergency consultation for epistaxis may be an unfavourable predictor of mortality [85]. Patients who are expected to maintain hemodynamic stability should be referred to an otolaryngologist for a more comprehensive medical evaluation and management [11]. The adoption of nasal endoscopes in the field of otorhinolaryngology has shifted the paradigm by which epistaxis is treated and has greatly improved outcomes. Endoscopes make it far easier to identify the point of bleeding with a high degree of accuracy [86]. It has been reported that without endoscopic assistance, clinicians fail to identify the point of bleeding in up to 50% of cases involving severe epistaxis [86].
Topical vasoconstrictors, such as oxymetazoline, phenylephrine, and lidocaine, have proven highly effective in the treatment of epistaxis [80,82]. Otolaryngologists spray a vasoconstrictor into the bleeding nostril and then insert a vasoconstrictor-soaked pledget into both nasal cavities and compress them tightly for 5 to 10 minutes before slowly removing the pledgets [80]. During nasal packing, it is important that the clinician inspect the oropharynx for signs of continuing bleeding.
In cases where direct pressure using vasoconstrictor-soaked pledgets is unsuccessful, chemical cautery can be attempted. This involves the application of a silver nitrate stick directly to the bleeding point for 10 to 20 seconds. Topical silver nitrate interacts chemically with the lining of the nasal mucosal, causing it to become inflamed. This leads to the excretion of fibrinous exudate, which coagulates on the surface to form a pseudomembrane that stops the bleeding [87]. Electrocauterization is also effective in dealing with persistent epistaxis of the anterior septum. A metallic hoop warmed by an electric circuit is placed around the bleeding artery, thereby enabling the application of heat to the affected area via radiation (i.e., without coming into direct contact). It is notable that the overuse of cauterization or the application of these techniques to both sides of the nasal septum can lead to septal perforation or other mucosal trauma, which may worsen the bleeding.
A failure to identify the bleeding point or stop the bleeding via cauterization is an indication that pressure should be applied directly at the site of epistaxis using special packing materials lubricated with antibiotic ointment. Several packing materials have been developed specifically for the treatment of epistaxis to overcome the difficulties involved in inserting conventional ribbon gauze (e.g., Vaseline or bismuth-iodoform paraffin paste impregnated packs). Two common pre-prepared packs on the market include non-absorbable Merocel (Medtronic Inc., Minneapolis, MN, USA) and absorbable Nasopore (Polyganics, Groningen, the Netherlands). In randomized, controlled trials, these materials were shown to stop the bleeding in approximately 60 to 90% of refractory cases [88-91].
Merocel is a sponge of hydroxylated polyvinyl acetate, which has been compressed through dehydration. Rehydration through the injection of normal saline causes it to increase in size within the nasal cavity, thereby compressing the bleeding point [92]. It also allows localized clotting factors to reach the concentration levels required for coagulation [87]. The downside of non-absorbable packing is the need to remove it, which can cause the patient considerable pain and discomfort. Nasopore is dissolvable, bioresorbable foam, which expands through the absorption of water to support the surrounding tissue and apply pressure to bleeding vessels in the nasal cavity. Nasopore can be suctioned from the nasal cavity after it begins to dissolve, usually within a few days of insertion [92]. Note that nasal packing material should always be inserted backwards along the roof of the mouth, instead of upwards. Incorrect insertion of these materials can exacerbate mucosal damage and worsen the bleeding. In the event that nasal bleeding continues after initial packing, additional packing on the opposite side of the nasal cavity is required.
Treating Posterior Epistaxis
Cases of posterior epistaxis are far less common and are generally referred to otolaryngologists for further management [26]. Difficulties in imaging and accessing the source of bleeding can greatly hinder treatment efforts. Several packing strategies have been devised for posterior epistaxis. In some cases, packing is meant only to stem the bleeding until a surgical solution can be implemented. The conventional approach involves the insertion of rolled gauze through the choana, which is then held in place in the oropharynx using silk stitches [45,93]. A number of companies have developed “posterior packs”, most of which involve the inflation of balloon catheters in the nasopharyngeal space to halt epistaxis. One alternative to posterior nasal tamponade is the insertion of a 10 to 14 French Foley catheter into the nasal passageway until it is visible in the oropharynx. The balloon is then filled with 10 to 15 mL of sterile water, whereupon the Foley catheter is retracted anteriorly until it is lodged against the posterior choana within the nasopharynx [11,45]. The catheter can be secured using a clamp before inserting an anterior pack.
Hot water irrigation is another approach to controlling posterior epistaxis [94-98]. A modified epistaxis-balloon-catheter is introduced into the bleeding nasal cavity to obstruct the choana. Continuous irrigation using 500 mL of hot water (50 ⁰C) is then applied for 3 minutes. This treatment is meant to decrease local blood flow by inducing mucosal edema. It is also meant to assist in clearing blood clots from the nasal cavity.
After packing, it is important to examine the oropharynx to determine whether posterior nasal bleeding has ceased. If the nasal packing proves effective, then it should be kept in place for 24 to 72 hours to enable time for healing prior to removal [7,25,45,99]. Maintaining nasal packing for longer than 72 hours increases the risk of complications, such as necrosis, toxic shock syndrome (fever, hypotension, desquamation, and mucosal hyperemia), sinus or nasolacrimal infections, and dislodgment [11].
Managing Conservative Treatment Failures
In cases where bleeding continues after packing, the patient should be immediately transferred to the emergency department for further management via arterial embolization or surgical intervention. For more than 30 years, embolization has proven to be a highly effective alternative to surgical ligation in the treatment of posterior epistaxis with success rates of approximately 90% [93]. The reported rate of severe complications, including stroke or blindness, ranges from 2 to 4% [93,100]. Before conducting an arteriogram, it is important to perform an otolaryngologic examination for the localization and/or lateralization of bleeding points. In the event that embolization fails to stop the bleeding, then surgical intervention is required.
When surgical intervention is deemed the only remaining option, the arteries to be targeted must first be identified based on physical examinations, endoscopic results, and the medical history of the patient. The conventional approach to the surgical ligation of ethmoidal vessels involves Lynch incisions [101]. Bipolar electrocoagulation is applied to clip or coagulate vessels after raising the periosteum off of the lacrimal crest and posteriorly into the orbit. Advances in endoscopy have also led to the development of endoscopic ligation techniques [102-104]. It is very likely that endoscopic artery ligation is a better treatment option for posterior epistaxis, due to its effectiveness and the fact that it is far less costly than is endovascular embolization [12]. Note that there may be an overlay between the right and left arterial systems, which can result in continuous nosebleeds despite unilateral arterial ligation.
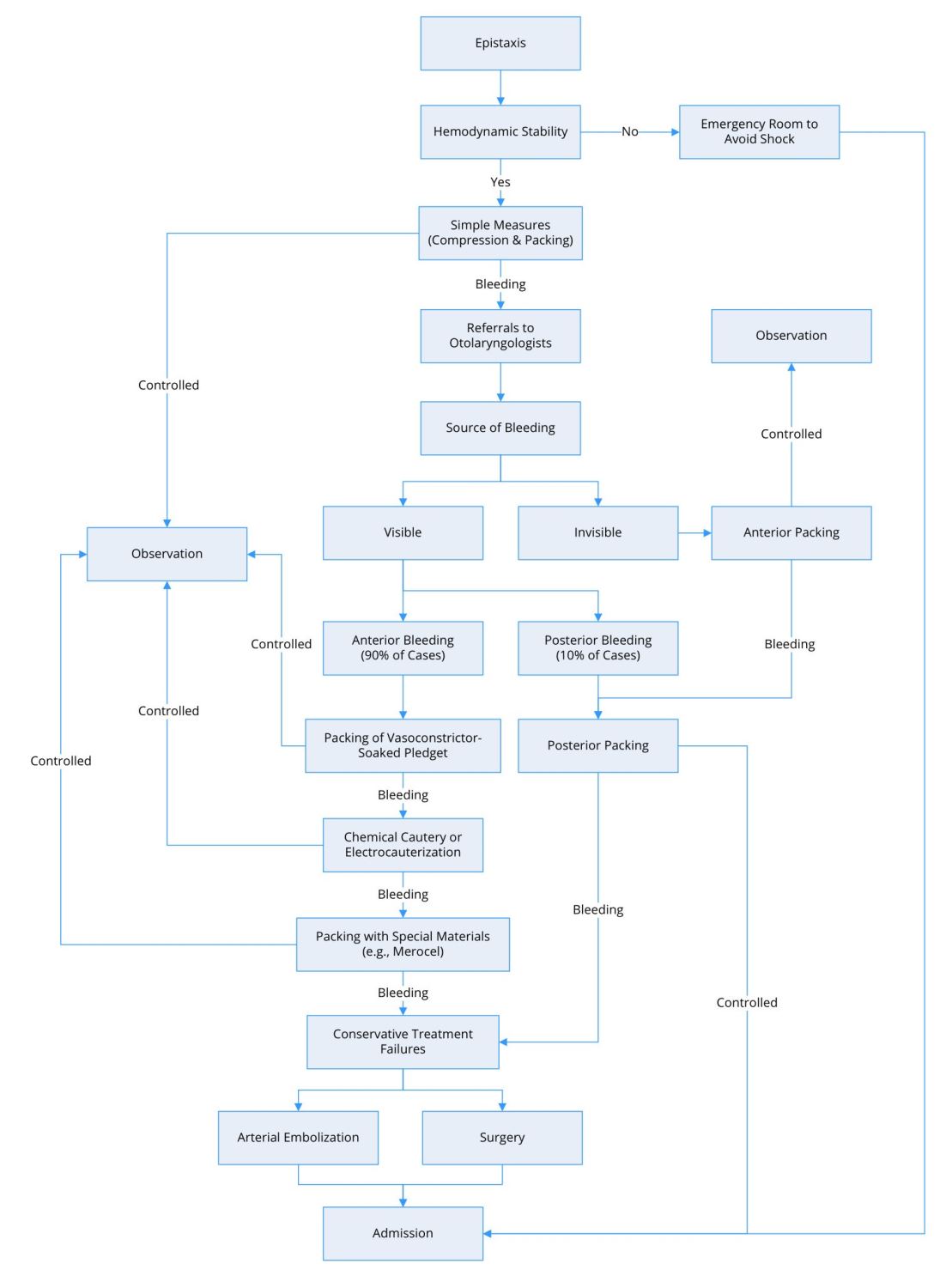
Figure 2. Flow diagram for the management of epistaxis.
Cauterization brings with it the risk of septal perforation, whereas packing can induce compression necrosis. The distress felt by patients undergoing these procedures can be alleviated using oral painkillers. Any packing method can result in a walled-off cavity in the sinuses, which greatly increases the risk of infection, toxic shock syndrome, or sinusitis [61]. Otolaryngologists therefore recommend the administration of prophylactic antibiotics with staphylococcal coverage, including amoxicillin-clavulanate or a second-generation cephalosporin [91,104-110].
There is, however, still some debate regarding the prescribing of prophylactic antibiotics for patients with nasal packing to prevent secondary bacterial sinonasal infection or toxic shock syndrome [11,111]. In practice, prophylactic antibiotics should be administered only to those facing a higher risk of infection, such as those who are immunosuppressed, have diabetes, or are of advanced age [11].
The dislodgement of posterior packing due to erroneous positioning can intensify vagal tone, resulting in bradycardia, hypotension, hypoventilation, or aspiration. It is therefore recommended that patients who undergo posterior packing be hospitalized and kept under observation [8,112]. Hospitalization may also be required for patients who have serious comorbidities, related symptoms, and/or intractable anterior nasal bleeding.
All patients who experience recurring epistaxis should be given rudimentary training in first-aid to deal with recurrences. They must first learn to apply pressure accurately in the cartilaginous region of the nose (rather than the nasal bridge) for at least 5 to 10 minutes. During compression, the patient should be sitting up and bent forward at the waist to minimize the risk of aspiration or the swallowing of blood into the oropharynx and stomach. The swallowing of excess quantities of blood can trigger a gag reflex and irritate the stomach that results in vomiting, which can exacerbate bleeding. Patients should refrain from hot foods, vigorous activities, and nose picking after treatment. It is also important to avoid nose blowing for 7 to 10 days after treatment [113].
Nasal saline washes, water-soluble creams, antiseptic creams, and/or petroleum jelly can help to keep the mucosa moist and assist in healing [112]. Clinical experience has shown that the application of generous quantities of petroleum jelly to the nostrils can prevent mucosal drying, which acts as a cost-effective solution for anterior nosebleeds. Researchers have demonstrated the efficacy of antiseptic creams for the treatment of persistent epistaxis in children as well as in adults [114]. Humidifiers are an effective preventive measure in dry environments, particularly during sleep. Parents should ensure that their children’s fingernails are clipped in order to reduce the damage from nose picking.
Numerous advances have been made in the management of epistaxis. Depending upon the suspected underlying cause of epistaxis and the equipment available at the primary care facility, practitioners may choose between conventional methods (e.g., nasal packing) and more sophisticated methods (e.g., electric cauterization and endoscopic devices). This article provides a useful flow diagram for the management of epistaxis to assist clinicians in clinical practice. There remains a dearth of high-quality research results by which to better formulate treatment algorithms aimed at optimizing outcomes.
Received date: September 18, 2018
Accepted date: October 31, 2018
Published date: March 12, 2019
© 2019 The Author. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The authors have addressed comments and concerns about a recent article, by Nasr et al, which reported the clinical characteristics and outcome of 34 patients with Takotsubo syndrome triggered by cerebral disease and collected during five years from a neurological ICU.
The authors report the first published case of a patient presenting with stridor from an acute, near complete, airway obstruction with normal biochemical markers, as the initial presentation of a parathyroid adenoma, which required immediate surgical intervention. The learning point from this case is to ensure a broad differential is placed forward when dealing with acute neck swelling. Routine Head and Neck pathology can present in an unusual fashion, with resultant challenging presentations.
The clinical significance of otitis media with effusion (OME), a complication associated with cleft lip/palate (CLP), is often overlooked in children. The author reviews the pathogenesis, clinical manifestations, and diagnoses of OME in children with CLP as well as the controversies surrounding treatment. He also provides a flowchart to guide the management of OME in children with CLP.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
This article presents a comprehensive review of schwannomatosis affecting cranial nerves, delineating its unique characteristics distinct from other forms of neurofibromatosis. By addressing diagnostic complexities and the evolving criteria for identification, the paper emphasizes the critical need for accurate recognition of schwannomatosis to facilitate effective management and provide essential genetic counseling. Enriched with a detailed case study, this review delivers vital insights into the epidemiology, symptomatology, and therapeutic strategies for schwannomatosis, advocating for a revision in current clinical approaches. This work is indispensable for medical professionals aiming to enhance diagnostic precision, comprehend genetic underpinnings, and improve patient outcomes. Offering a thorough analysis of this rare condition, the article is pivotal not only for clinicians and researchers in the neurogenetic field but also for a broader spectrum of medical and scientific communities, bridging a notable gap in contemporary medical literature.
The author reframes review articles as evidence infrastructure that updates clinical consensus and identifies research gaps. The author argues that narrative reviews integrate findings across biological scales and translate molecular data into clinically meaningful explanations of disease behavior. Using cholesteatoma, the author illustrates how molecular evidence can recast invasiveness and recurrence into a mechanistic model that also motivates the pursuit of nonsurgical therapies. The author contrasts this interpretive role with systematic reviews, which function as reproducible decision chains grounded in transparent, verifiable methodology. Finally, the author cautions that AI assisted automation can propagate error unless it is governed by expert oversight within a human in the loop model that preserves auditable decision chains.
Otolaryngologists and gastroenterologists seem to differ in their definitions and management of laryngopharyngeal reflux (LPR). In this review article, the author suggests a multidisciplinary approach to LPR diagnosis. Based on the latest findings, the author proposes an algorithm to facilitate the assessment and management of LPR.
Kuo CL. Updates on the management of epistaxis. Clin Med Ther 2019;1(1):5. https://doi.org/10.24983/scitemed.cmt.2019.00106