Objective: This review aims to consolidate existing knowledge about schwannomatosis in cranial nerves and to detail the evolution of diagnostic criteria for this rare variant of neurofibromatosis. To our knowledge, this represents the inaugural comprehensive literature review focusing on this less recognized neurofibromatosis form.
Methods: We performed a thorough search of the PubMed database, managed by the National Library of Medicine, reviewing literature from May 1984 through January 15, 2024. Our search criteria included various combinations of terms related to each cranial nerve, along with "multiple," "schwannoma," "neurilemmoma," "nerve sheath tumor," "Schwann cell tumor," "Schwannomatosis," and "Neurofibromatosis type 3." This extensive review is further complemented by a case study of a 58-year-old Chinese woman with a previous diagnosis of nasopharyngeal carcinoma, who presented with a cervical mass. Histological analysis confirmed this mass as schwannomatosis of the vagus nerve, distinguished by multiple fusiform enlargements.
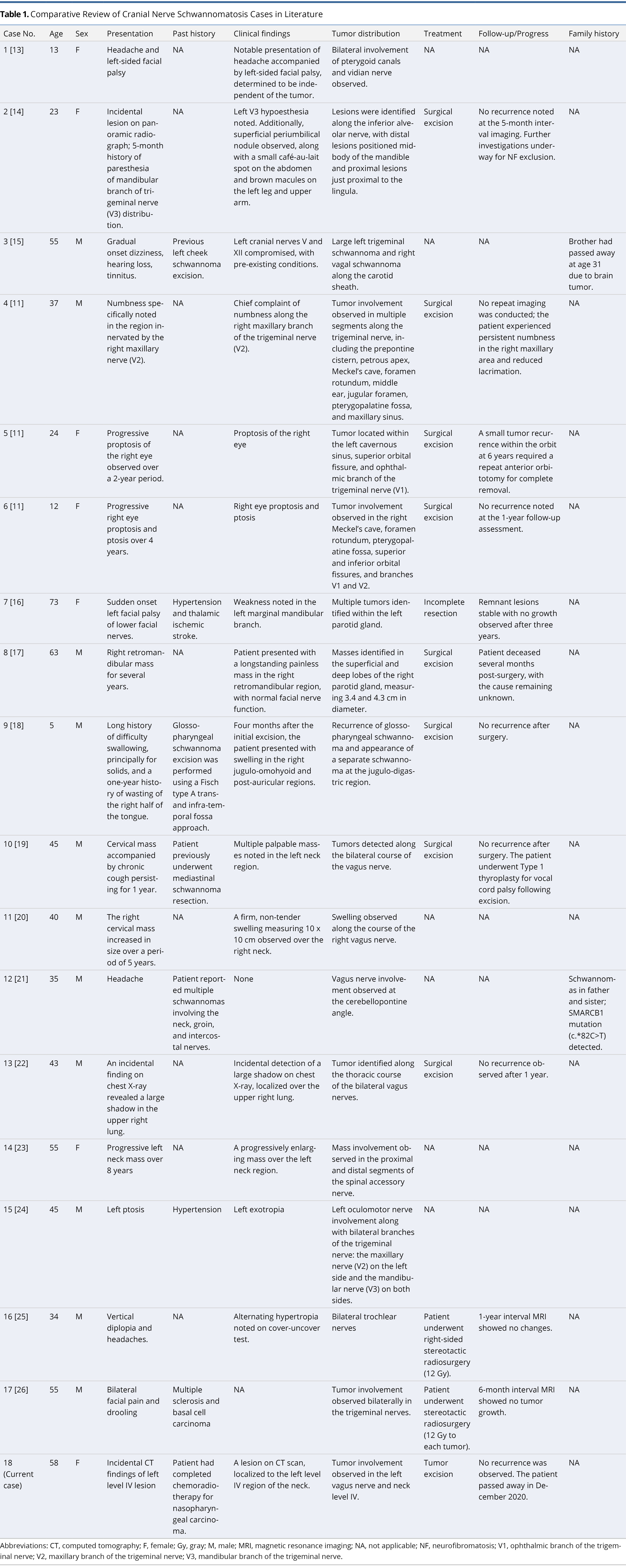
Results: We identified fifteen articles that, along with our case report, discuss a total of 18 cases of cranial nerve schwannomatosis. The distribution of cases includes the trigeminal nerve (7 cases), vagus nerve (6 cases), facial nerve (3 cases), and single cases involving the oculomotor, trochlear, glossopharyngeal, and spinal accessory nerves. Two of the cases featured concurrent involvement of multiple nerves. Predominantly reported in males, the diagnosis is most frequently made between the fifth and sixth decades of life. Only one case reported a positive family history of the SMARCB1 mutation. Recurrence occurred in one patient, and no cases of malignant schwannomas were reported.
Conclusion: Schwannomatosis should be considered in the differential diagnosis for patients presenting with multiple schwannomas or extensive involvement of a single nerve, especially those not meeting the criteria for neurofibromatosis type 2. The implications for malignancy risk, surveillance, genetic testing, and counseling are significant and distinct from other forms of neurofibromatosis. As diagnostic criteria continue to evolve, it is crucial for clinicians to stay updated with the latest developments in the field.
Schwannomatosis, often referred to as neurofibromatosis type 3, represents a rare but significant category within the broad array of neurofibromatosis disorders. These genetic conditions are notable for their predisposition to fostering the development of neurogenic tumors. In contrast to neurofibromatosis type 2, which is known for its association with vestibular nerve tumors, neurofibromatosis type 3 is predominantly characterized by the proliferation of multiple schwannomas along the cranial, spinal, and peripheral nerves, notably sparing the vestibular nerve [1,2].
Previously considered a mere variant of neurofibromatosis type 2, schwannomatosis was recognized as a distinct entity in 1996 when molecular tumor analyses unveiled mutations in the SMARCB1 and LZTR1 genes. This crucial breakthrough prompted the establishment of specialized diagnostic criteria for schwannomatosis in 1997, criteria which have been continually refined [3]. Schwannomas, the signature tumors of schwannomatosis, are generally benign, slow-growing, encapsulated growths that originate from Schwann cells. These cells are vital for the myelination of peripheral, cranial, and autonomic nerves. Although typically solitary, the presence of multiple schwannomas signals potential schwannomatosis, necessitating deeper investigation. With an estimated prevalence of 0.58 cases per million, this condition remains exceedingly rare [4,5]. The pathogenesis of schwannomatosis adheres to a “four-hits, three-step” model where a mutation in the remaining healthy gene sparks localized schwannoma growth [6,7]. Predominantly, schwannomatosis affects peripheral nerves, seen in 89% of cases, and spinal nerves, which are involved in approximately 74% of instances [1]. Although less common, when cranial nerves are affected, the trigeminal nerve is most frequently implicated [1,8,9]. This highlights the distinct patterns of nerve involvement in schwannomatosis, providing critical insights into the nature of this rare neurofibromatosis variant.
This paper provides a detailed analysis of a definitive case of vagus nerve schwannomatosis and offers an extensive literature review on the occurrence of this condition in cranial nerves. Since its separation from neurofibromatosis type 2 in 1996, research on cranial nerve schwannomatosis has been limited. Our study contributes critical insights into the clinical features and management of this rare disorder, pinpointing substantial research gaps. Additionally, we explore the evolution of diagnostic criteria and recent advancements in the field, aiming to enhance understanding and direct future research in diagnosing and managing schwannomatosis. This effort is essential for enriching clinical practice and propelling the research landscape forward in this specialized area.
Ethical Standards in Retrospective Analysis
In this retrospective analysis, we reviewed electronic medical records and archived histological slides from a patient treated by the senior author at our institution. Approval from the Institutional Review Board (IRB) was not required per our institutional guidelines; however, written informed consent was secured from the patient, ensuring adherence to ethical standards.
Research Protocol for Literature Review
For our literature review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, as detailed in Figure 1. We conducted a detailed search of the PubMed database, which is managed by the National Library of Medicine, spanning literature from May 1984 to January 15, 2024. Our search strategy employed both text words and Medical Subject Headings (MeSH) to facilitate a structured and thorough exploration of the literature.
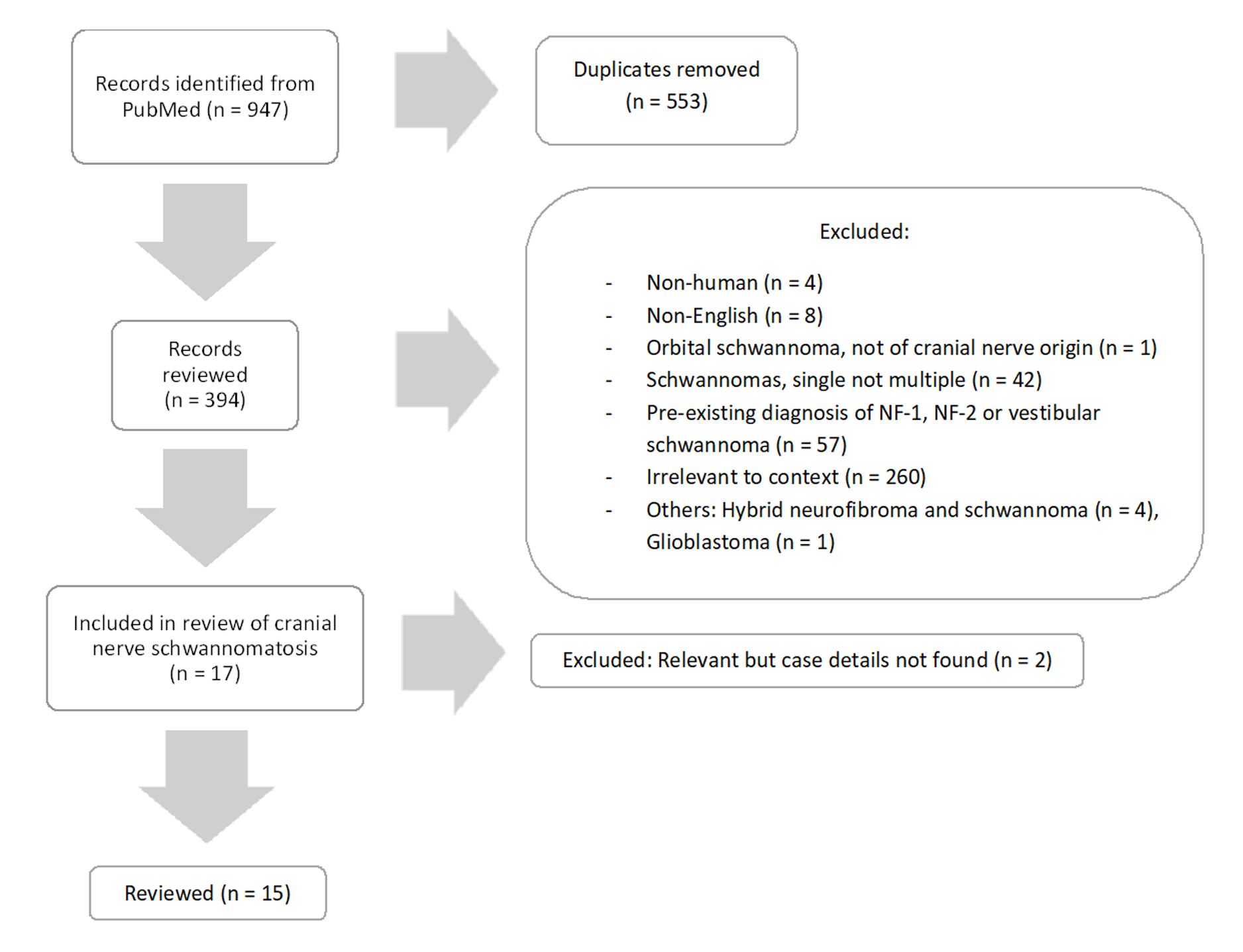
Figure 1. Systematic selection flowchart according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. This flowchart graphically details the methodical approach of conducting a literature review, starting with an extensive search in the PubMed database (947 articles identified). The process rigorously applies the PRISMA standards, filtering out duplicates (553 articles removed) and excluding studies not meeting specific criteria related to language and cranial nerve origin (377 articles excluded). The focus narrows to 17 articles directly related to cranial nerve schwannomatosis, with 15 articles ultimately meeting all criteria for thorough review.
Search Strategy
We crafted our search strategy to include specific terms for each cranial nerve by name and number, such as “olfactory nerve” and “Cranial nerve 1.” These terms were linked with keywords that indicate the presence of multiple tumors or describe specific types of tumors, using the Boolean operator “AND.” The keywords we used included “multiple,” denoting more than one tumor; “schwannoma” and “neurilemmoma,” both referring to types of benign tumors that arise from nerve sheath cells; “nerve sheath tumor,” a broad term for tumors that develop from the protective coverings of nerves; and “Schwann cell tumor,” specifically relating to tumors originating from Schwann cells, which are essential for nerve fiber insulation outside the central nervous system.
Our selection criteria were guided by the clinical diagnostic criteria for schwannomatosis, as revised in 2006 by Baser et al. [10]. This approach was preferred over more recent molecular diagnostic techniques to ensure that our review focused on clinically relevant data, providing a comprehensive overview of the condition’s diagnostic challenges and characteristics.
Study Selection Process
Authors JYL and ML initially identified 947 records, from which 553 duplicates were removed, leaving 394 studies for abstract review. Of these, 17 articles underwent full examination. Any discrepancies were resolved through consultation with the senior author, HXY.
Exclusion Criteria
Of the studies reviewed, 377 were excluded for various reasons: four discussed non-human subjects, eight were not in English, 260 did not pertain directly to our topic of interest (covering intra-operative neuromonitoring, radiological evaluations, and post-operative neurological management), and 99 did not meet the criteria for schwannomatosis (42 did not involve multiple schwannomas, and 57 were related to neurofibromatosis types 1 or 2, or vestibular schwannomas). Additionally, one case of orbital schwannoma was excluded for not originating from a cranial nerve, four were excluded for discussing hybrid neurofibromas, and one for involving a glioblastoma.
Final Study Inclusion
Ultimately, 15 studies satisfied all inclusion criteria and were selected for our comprehensive review. Within these studies, individual cases were meticulously analyzed and subjected to the previously mentioned exclusion criteria. Notably, this process identified a unique case involving a hybrid neurofibroma and schwannoma [11]. This selective approach ensures a focused and detailed examination of relevant literature, contributing to a robust understanding of schwannomatosis.
Case Report
A 58-year-old Chinese woman previously treated for nasopharyngeal cancer presented with a left neck mass during routine surveillance scans. At 53, she was diagnosed with left-sided T1N3bM0 undifferentiated non-keratinizing squamous cell nasopharyngeal carcinoma and underwent concurrent chemotherapy and radiotherapy. She lacked a family history of neurocutaneous tumors and showed no neurofibromatosis-related physical signs such as café-au-lait spots or Lisch nodules. Post-treatment in 2013, she remained compliant with follow-ups and showed no signs of disease recurrence.
Diagnostic procedures
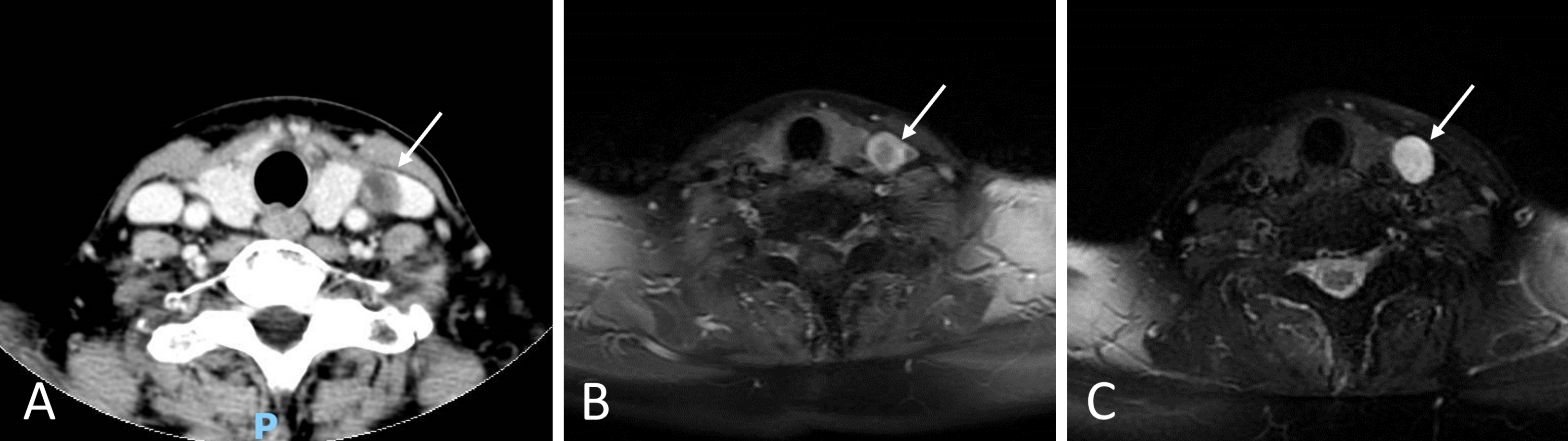
Five years after completing treatment, concerns regarding the effacement of the right Fossa of Rosenmüller emerged, necessitating further diagnostic imaging. Enhanced computed tomography (CT) scans of the neck revealed a heterogeneously enhancing lesion on the left side (Figure 2A). Subsequent magnetic resonance imaging (MRI) studies of the neck, using T1-weighted post-contrast techniques, identified a contrast-enhancing lesion adjacent to the left thyroid lobe (Figure 2B). T2-weighted MRI images further characterized the left neck lesion as heterogeneously hyperintense, with well-defined, circumscribed margins (Figure 2C).

Figure 2. Imaging of the left neck lesion. (A) A contrast-enhanced CT scan of the neck shows a left-sided neck lesion (indicated by a white arrow), which displays heterogeneous enhancement with contrast material. (B) A T1-weighted, post-contrast MRI of the neck reveals a lesion (white arrow) with contrast enhancement situated laterally to the left thyroid lobe. (C) On a T2-weighted MRI of the neck, the same lesion (white arrow) is seen as heterogeneously hyperintense and appears to have a well-circumscribed boundary with clearly defined margins.
Despite these findings, positron emission tomography (PET) scans showed no significant 18-fluorodeoxyglucose (FDG) uptake; however, increased FDG activity was observed in the right postnasal space. The mass raised suspicions of a centrally attenuated lymph node, which could potentially indicate nodal recurrence, especially given the patient’s history of cancer. However, a biopsy from the postnasal space returned negative for malignancy, and fine needle aspiration of the left level IV mass was non-diagnostic. Additional MRI scans covering the internal acoustic meatus and skull base revealed no evidence of vestibular schwannomas.
Surgical intervention
Subsequent surgical interventions revealed a 2 cm fusiform cystic mass between the thyroid gland and internal jugular vein, contiguous with the vagus nerve. Histology from a frozen section suggested a schwannoma. Deep biopsies down to the prevertebral fascia showed no tumor recurrence, and the vagus nerve was reconstructed with a greater auricular nerve cable graft.
Histological analysis
Histological analysis of the vagus nerve specimen revealed multiple discrete fusiform enlargements with spindle cells in Antoni A and Antoni B areas, diagnostic of schwannomas and positively stained for S-100, contrasting with the regular morphology of uninvolved nerve fibers (Figure 3). The schwannomas tested negative for CD34 and AE1/3. With multiple schwannomas confirmed and no evidence of vestibular involvement, the diagnosis was schwannomatosis.

Figure 3. Histopathological characteristics of schwannoma. (A) The image presents tightly packed spindle cells and the characteristic Verocay bodies, indicative of schwannoma (hematoxylin-eosin stain, 20x). (B) The view exhibits spindle cells with distinct nuclear palisading around Verocay bodies, a hallmark of schwannoma (hematoxylin-eosin stain, 20x). (C) The field shows the juxtaposition of hypercellular Antoni A areas with the myxoid, hypocellular Antoni B regions, typical in schwannoma (hematoxylin-eosin stain, 2x). (D) The section depicts a well-defined nodule of spindle cells, illustrating the variation in cellularity that includes regions characteristic of both Antoni A and B patterns, consistent with schwannoma (hematoxylin-eosin stain, 20x).
Postoperative outcome and follow-up
Following the surgical procedure, the patient experienced a postoperative complication characterized by paralysis of the left vocal cord. Subsequent monitoring revealed no evidence of disease recurrence. The patient’s condition remained stable in this context until her death in December 2020, which was attributed to a distinct medical condition unrelated to the original diagnosis.
Literature Review
Our literature review identified 16 relevant papers [11–26]. However, one paper [12], which detailed two cases involving the superior orbit, was excluded. It did not directly pertain to the optic nerve and therefore was not considered indicative of cranial nerve schwannomas. Consequently, a total of 15 papers described schwannomatosis of the cranial nerves (Table 1). Of these, fourteen were single-case reports, and one was a case series that described three cases, collectively accounting for 17 cases of cranial nerve schwannomatosis. Including our reported case, 18 cases of cranial nerve schwannomatosis were analyzed. The distribution was as follows: trigeminal nerve (7 cases), vagus nerve (6 cases), facial nerve (3 cases), and then one case each involving the oculomotor, trochlear, glossopharyngeal, and spinal accessory nerves. Two cases exhibited concurrent involvement of more than one nerve: one case involved both the trigeminal and vagus nerves, and another involved both the oculomotor and bilateral trigeminal nerves. There were no reports of schwannomatosis affecting the abducens or hypoglossal nerves.
Demographics and clinical presentation
Schwannomatosis was more frequently reported in males [11] compared to females [7]. The age at diagnosis for cranial nerve schwannomas ranged from 5 to 73 years, with the majority of diagnoses occurring between the fifth and sixth decades of life. Only one of the cases (Case 12) had a confirmed positive family history with an SMARCB1 mutation. Additionally, Case 3 involved a patient whose brother had a history of a brain tumor, which may be related to his diagnosis of schwannomatosis, though the information available is insufficient for a definitive conclusion. Clinical manifestations of the disease varied and included symptoms such as headaches, facial palsy, numbness, and proptosis. Medical histories of the patients were diverse, with clinical findings often including facial palsy, hypoesthesia, and cervical masses. The tumors were located in areas such as the pterygoid canals, cavernous sinus, and parotid gland.
Treatment and histological findings
In 15 cases, the schwannomas were surgically removed, and the histological analysis consistently identified characteristics of schwannomas, such as palisading nuclei along with Antoni A and Antoni B tissue patterns. All specimens tested positive for S-100 protein on immunohistochemical staining and showed no signs of malignancy. Genetic analysis was conducted in only one case (Case 12), prompted by a significant family history, which identified a germline mutation (c.*82C>T) in SMARCB1. Two other cases underwent stereotactic radiosurgery without surgical excision, while the treatment approach was not reported in one case.
Recurrence and follow-up
Recurrence was reported in one of the 18 patients (Case 5), occurring approximately six years after the initial resection. Another case (Case 7) involved incomplete resection; however, no growth of the residual tumor was observed at the three-year mark. Five cases showed no recurrence after a monitoring period ranging from a minimum of five months to a maximum of six years (Cases 2, 6, 9, 13, and 15). Six cases had no reported surveillance for recurrence (Cases 1, 3, 10, 11, 12, and 14), and in two cases, the patients either defaulted on follow-up or demised (Cases 4 and 8). None of the 18 patients were diagnosed with malignant schwannomas.
Schwannomatosis, a rare hereditary genetic disorder, is cataloged under the code 162091 in the Mendelian Inheritance in Man (MIM) database. This review is notably the first to focus exclusively on cranial nerve schwannomatosis, underscoring its significance in medical research and clinical practice. Understanding this disorder is critical, especially for determining how frequently patients should be monitored and the criteria for intervention, whether surgical or otherwise. We have analyzed 18 cases documented in the literature that specifically address schwannomatosis affecting cranial nerves.
Among these cases, the trigeminal and vagus nerves were identified as the most commonly impacted. This finding deviates from the usual prevalence of schwannomas, where vestibular schwannomas dominate, followed by those affecting the trigeminal, facial, glossopharyngeal, vagus, and spinal accessory nerves. The unique pattern of nerve involvement observed in cranial nerve schwannomatosis could be attributed to the limited number of cases available for study, which may not provide a robust enough sample size to accurately mirror true nerve involvement. Furthermore, the exclusion of three non-English reports [27–29] concerning schwannomatosis of the trigeminal and facial nerves might have influenced the observed distribution of nerve involvement, suggesting potential underreporting or selective documentation in available literature.
Unique Schwannomatosis Case Presentation
Our case study highlights a 58-year-old Chinese woman diagnosed with schwannomatosis of the vagus nerve, which is recognized as the second-most commonly affected cranial nerve in such conditions. This case is distinguished by its unique circumstances: the patient had previously undergone chemoradiotherapy for nasopharyngeal carcinoma, and the schwannomatosis was incidentally detected during a routine evaluation for possible malignancy recurrence.
This differs from other reported instances of vagus nerve schwannomatosis, such as Cases 3 and 10, where patients typically presented with palpable neck masses. In contrast, our patient had no prior significant medical history of schwannoma-related lesions and showed no symptoms attributable to the schwannoma. The lesion was surgically removed primarily to rule out the possibility of nodal recurrence of the nasopharyngeal cancer, even though the patient was asymptomatic. This scenario underscores the incidental nature of some schwannomatosis diagnoses, particularly in individuals with complex oncological histories, and highlights the importance of diligent differential diagnosis in post-cancer surveillance.
Olfactory Nerve Involvement in Schwannomas
Traditionally, olfactory nerves are thought to be exempt from schwannomatosis due to their lack of a myelin sheath. Despite this, there are documented case reports of olfactory schwannomas [30,31], though these do not meet the established criteria for schwannomatosis. Kim DY et al. [31] have introduced two theories to explain these rare occurrences. The developmental hypothesis suggests that mesenchymal pial cells might migrate into ectodermal Schwann cells within the central nervous system. Alternatively, the non-developmental hypothesis argues that olfactory schwannomas could stem from adjacent structures, such as the perivascular nerve plexus. Additionally, Murakami et al. [30] theorize that the origin of such schwannomas may be the nerve sheath located approximately 0.5 mm beyond the olfactory bulb, proposing a potential anatomical site for these unusual schwannomas.
Optic Nerve Involvement in Schwannomas
Theoretically, schwannomas of the optic nerve are considered improbable, as these nerves are myelinated by oligodendrocytes rather than Schwann cells. However, a hypothesis suggests that the rare instances of schwannomas may arise from small sympathetic fibers that innervate the surrounding vasculature [32]. Our review identified several cases of orbital schwannomas [12], but it remains uncertain whether these tumors originated directly from the optic nerve or from other nearby cutaneous or autonomic nerves. This ambiguity highlights the complexity of diagnosing and understanding schwannomas in regions where multiple nerve types coexist.
Recurrence and Surgical Techniques
Recurrence was documented in only one of our 18 cases (Case 5), approximately six years after the initial surgical resection. This instance suggests that the overall rate of recurrence might be underestimated within this cohort, given that most cases had not yet reached such an extended duration of follow-up at the time of our study’s publication. Additionally, many reports lacked specific surgical details, including whether procedures involved intracapsular excision or complete excision of the nerve of origin. This omission is crucial because intralesional resection is known to have a fourfold higher risk of recurrence compared to en bloc resection, where the entire nerve segment is removed [33]. The lack of detailed surgical data highlights the need for thorough documentation in clinical practice to better understand and mitigate the risk of schwannoma recurrence.
Malignant Transformation Risks
In our review, none of the cases demonstrated features indicative of malignant transformation. However, it is important to note that while the risk of malignant transformation in schwannomas not linked to genetic syndromes is typically less than 1% [34], this risk could be higher in cases associated with neurofibromatosis type 3, though the exact level of increased risk is not well-defined. Moreover, schwannomatosis involving the SMARCB1 mutation may present an even greater risk of malignant transformation [35]. These considerations highlight the complexity of managing schwannomatosis, emphasizing the need for vigilant monitoring and tailored therapeutic approaches based on genetic background and individual risk factors.
Evolution of Diagnostic Criteria and Recent Updates
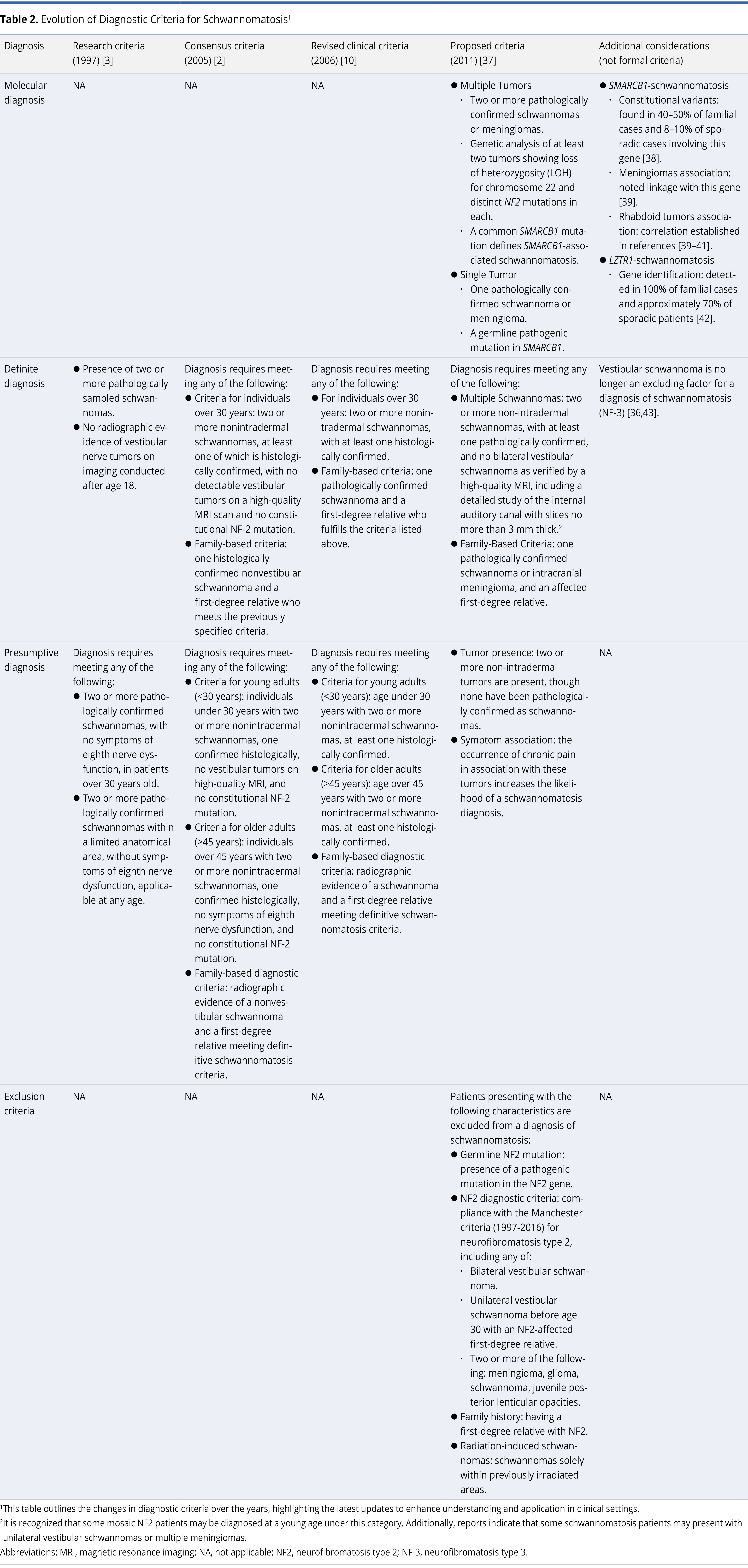
Since 1996, schwannomatosis and neurofibromatosis type 2 have been recognized as distinct disorders, each defined by unique clinical features [3]. Neurofibromatosis type 2 is catalogued in the Mendelian Inheritance in Man (MIM) database under the identifier 101000. This MIM number is a unique code used to classify genetic disorders, helping to provide clear, accessible information about their genetic basis and clinical characteristics. Over the years, the diagnostic criteria for schwannomatosis have undergone significant evolution, as detailed in various studies (Table 2) [2,3,10,36–43].
Initially, in 2005, MacCollin et al. proposed diagnostic criteria that excluded the presence of vestibular schwannomas, even in unilateral cases [2]. Despite this exclusion, individuals with schwannomatosis still face a risk comparable to the general population for developing vestibular schwannomas, particularly after age 50 [10,18,44]. However, the occurrence of bilateral vestibular schwannomas before age 50 typically suggests neurofibromatosis type 2 [45]. Recent updates have refined the diagnostic criteria for schwannomatosis to exclude bilateral vestibular schwannomas, following observations of such tumors in both LZTR1-schwannomatosis [36] and non-LZTR1 schwannomatosis [46]. These changes highlight the critical role of genetic analysis in patients presenting with multiple schwannomas [37].
MacCollin et al. categorized schwannomatosis into two distinct types. Familial schwannomatosis is identified by the presence of one or more schwannomas in a first-degree relative. Conversely, sporadic schwannomatosis occurs in individuals who do not have affected family members [2,47]. Unlike neurofibromatosis type 2, patients with schwannomatosis do not show germline NF2 gene mutations but instead display different somatic variants across multiple tumors [48]. Inheritance patterns remain unclear, with sporadic schwannomatosis accounting for the majority of cases and familial schwannomatosis for 15–25% [37].
Genetic studies have identified two predisposing genes, SMARCB1 and LZTR1, both located on chromosome 22q, centromeric to NF2. Notably, mutations in SMARCB1 occur in 40–50% of familial schwannomatosis cases and 8–10% of sporadic schwannomatosis cases [38], while mutations in LZTR1 are present in all familial schwannomatosis cases and 70% of sporadic schwannomatosis cases [37]. Overall, mutations in SMARCB1 and LZTR1 are found in 70–85% of familial schwannomatosis cases and in 30–40% of sporadic cases, demonstrating a significant overlap with mosaic neurofibromatosis type 2 [35]. Evans et al. have noted that 37% of de novo cases meeting the criteria for schwannomatosis could be attributed to mosaic neurofibromatosis type 2 [49]. This high incidence of mosaicism, along with issues of variable penetrance, emphasizes the necessity for comprehensive genetic studies. Such analyses are crucial not only for assessing the risk of malignant transformation [9,50,51], but also for identifying related manifestations in tumor syndromes.
ERN GENTURIS Management Guidelines
In April 2022, the European Reference Network on Genetic Tumour Syndromes (ERN GENTURIS) released detailed recommendations for managing schwannomatosis [35]. Although these guidelines broadly cover schwannomatosis, they are particularly relevant to cranial nerve schwannomatosis and offer substantial guidance. The guidelines prioritize the management of functionally significant schwannomas and those with potential for malignant transformation, emphasizing the importance of tailored treatment plans.
Diagnostic and surveillance protocols
The preferred imaging modality for early detection and management planning is MRI rather than PET scans. It is recommended that a comprehensive craniospinal MRI be conducted as a baseline in late childhood to facilitate timely diagnosis and effective intervention strategies. This approach ensures detailed visualization of cranial and spinal structures, which is crucial for accurate assessment and proactive management.
Follow-up MRIs are typically recommended every 2 to 3 years, based on clinical judgment, with more frequent monitoring needed if symptoms intensify or evolve. Additionally, MRI scans of the internal acoustic meatus with fine cuts are essential at the initial diagnosis to exclude bilateral vestibular schwannomas, indicative of neurofibromatosis type 2. However, the presence of a unilateral vestibular schwannoma does not rule out schwannomatosis, especially in cases associated with the LZTR1 variant, necessitating nuanced diagnostic assessments.
El Sayed et al. have identified tumors with growth rates exceeding 2 cm3 per year or a relative growth rate of more than 35% per year as indicative of rapid growth [52]. Despite this finding, ERN GENTURIS does not consider rapid growth alone as a criterion for intervention. Yet, in the context of cranial nerve schwannomatosis, particularly for intracranial lesions that might lead to mass effects, rapid growth should trigger consideration for intervention.
Challenges and treatment considerations
While schwannomatosis affecting spinal nerves can lead to motor sensory dysfunction, involvement of cranial nerves often introduces additional complexities such as extrasensory dysfunction and compressive symptoms. Therefore, addressing only the symptom of pain in cases of cranial nerve schwannomatosis is insufficient. The broader spectrum of functional deficits caused by cranial nerve involvement presents unique clinical challenges, which may not consistently respond to interventions recommended by ERN GENTURIS’s guidelines.
Although the latest clinical practice guidelines generally advise against the use of radiotherapy due to the associated risk of malignant transformation, there are circumstances where radiotherapy could play a critical role, especially when surgical options are limited or if the schwannoma is in a location that complicates traditional surgical approaches. In such cases, radiotherapy, including advanced modalities like proton beam therapy, might be considered as a viable alternative, emphasizing the need for a balanced, case-by-case evaluation to optimize treatment outcomes in cranial nerve schwannomatosis.
Surgical and Alternative Treatments
Schwannomas can be managed through various approaches depending on their symptoms and locations. While pharmacologic therapy offers a conservative treatment route, surgical excision is typically the preferred option for symptomatic schwannomas [37]. Intracapsular enucleation is often favored to minimize damage to surrounding tissues, but extracapsular resection or tumor debulking might be necessary in cases where the tumor’s anatomy is less favorable.
For situations where surgery is not feasible or the patient opts against it, radiotherapy [53] and proton beam therapy [54] present viable alternative treatments for managing cranial nerve schwannomatosis. However, these therapeutic options carry a potential risk of malignant transformation [35], which necessitates careful consideration and discussion with the patient about the benefits and risks associated with each treatment modality. This comprehensive approach allows for personalized treatment plans that best suit the individual needs and conditions of patients with schwannomas.
Clinical Practice Implications
Schwannomatosis, though rare, should be a consideration for clinicians who encounter patients with multiple schwannomas or longitudinal involvement of a single nerve showing multiple discrete fusiform enlargements. Recognizing this condition is crucial as it significantly impacts clinical decisions. Typically, isolated schwannomas require minimal follow-up after excision due to their low risk of recurrence, malignant transformation, or spreading to other sites [55]. However, these characteristics can lead to potential misdiagnosis if not adequately recognized.
An understanding of the risks associated with schwannomatosis is essential for setting appropriate surveillance intervals and methods [35,56,57]. The criteria for surgical intervention and the adoption of other treatment strategies, such as radiotherapy, may vary in cases with genetic predispositions [58,59]. While chemotherapy has a limited role in the treatment of schwannomatosis [60,61], immunotherapy options such as Bevacizumab may be considered for cases that are not amenable to surgery [35].
Additionally, integrating genetic testing and counseling into the management strategy is vital [35,47]. These genetic insights not only inform treatment decisions but also provide crucial information on familial risk, aiding in the management and preventive strategies for affected families. This comprehensive approach ensures that all aspects of schwannomatosis are addressed, leading to informed and effective management of the condition.
Study Limitations
The primary limitation of our review is the inherently small sample size, a typical challenge in research involving rare diseases. However, this limitation also underscores the value of the cases we have presented, as each addition to the literature enriches the data pool available for future statistical analysis of this topic. Furthermore, the absence of genetic testing in most reported cases, including our own, marks a significant deficiency. This gap is often due to the high costs and limited availability of genetic testing. Among the 18 cases reviewed, six lacked any reported follow-up, and the duration of follow-up varied significantly, ranging from as short as five months to as long as six years. This constrained follow-up period potentially leads to an underestimation of the risks associated with recurrence and malignant transformation in schwannomatosis. Addressing these gaps in genetic insights and follow-up data is crucial for accurately characterizing the long-term outcomes and guiding the development of more effective management strategies for this complex condition.
Schwannomatosis should be considered in patients presenting with multiple schwannomas or extensive longitudinal involvement of a single nerve with multiple discrete fusiform enlargements, particularly when they do not meet the criteria for neurofibromatosis type 2. It is crucial to stay updated on the evolving diagnostic criteria for schwannomatosis, as changes can significantly impact clinical management. Further genetic testing is essential to differentiate between germline and somatic mutations, providing insights into the genetic basis of the condition. Additionally, a thorough review of family history is necessary to assess potential malignancy risks, which will guide the frequency of screening and the approach to genetic counseling. This comprehensive assessment helps develop tailored management plans that address both immediate and long-term health considerations for individuals with schwannomatosis.
Received date: December 03, 2023
Accepted date: February 21, 2024
Published date: May 20, 2024
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)’ autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2024 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
Delirium is an acute disorder of arousal and attention that is commonly encountered, incompletely understood, and associated with adverse outcomes including increased morbidity and mortality, reduced health-related quality of life, and increased healthcare costs. In this narrative review, authors examine the epidemiology, potential pathophysiological mechanisms, assessment, prevention, and treatment of this cognitive disorder.
ICG lymphography is an invaluable tool in lymphedema management. Both immediate and delayed scans are needed when performing the study. The delayed scan needs to be performed at the time of the lymphographic plateau to appreciate the full extent of the pathology. Using a recumbent cross trainer, the lymphographic plateau can be achieved in 15 minutes following ICG injection. We have found this exercise enhanced ICG lymphography protocol worthwhile of adoption by high volume lymphedema centers to raise diagnostic accuracy and efficiency.
The article stands out by providing a comprehensive analysis of a rare case of a midline branchial cleft cyst, a significant contribution given that only three other cases have been documented in medical literature. This scarcity underscores the article's value, making it a crucial read for medical professionals. It not only highlights the diagnostic challenges and treatment strategies for such atypical presentations but also enriches the understanding of branchial cleft cysts beyond the common lateral neck occurrences. By including a detailed review and comparative analysis of these few reported cases, the article offers unique insights into the demographic, symptomatic, and anatomical variations of branchial cleft cysts. This focused analysis makes the article an indispensable resource for clinicians, surgeons, and students in the medical field, aiming to enhance diagnostic accuracy, inform clinical practice, and ultimately improve patient outcomes in dealing with complex and rare presentations of congenital anomalies.
AI is rapidly evolving from a supportive tool into a core component of medical decision making and evidence synthesis, reshaping how clinicians interpret information at the point of care. Yet, while much of medical AI research emphasizes algorithmic performance and explainability, it seldom addresses the more practical question: how should physicians evaluate an AI recommendation in real-world, high-risk situations when fluent outputs can conceal critical errors. This Perspective offers a clinician-centered framework that treats AI outputs as provisional, testable hypotheses rather than definitive conclusions. By guiding users through premise verification, terminological precision, evidence appraisal, and causal analysis, it provides a structured defense against hallucinations, selective reporting, and data poisoning, using otolaryngology as a high-stakes, multimodal model. By placing clinical judgment at the center of AI use, this work shifts the field from passive automation toward safer, more accountable decision support grounded in patient safety.
This article presents a comprehensive discussion of advanced techniques in managing pediatric airway obstructions caused by vallecular cysts. By employing awake fiberoptic intubation and transoral CO₂ laser microsurgery, the authors highlight a thoughtful, evidence-based approach that emphasizes both safety and precision. While not groundbreaking, the depth of analysis in the decision-making process and procedural techniques offers invaluable insights for clinicians, particularly in pediatric otolaryngology. The article serves as a critical reference for handling complex airway cases, balancing innovative practices with established methods. Its significance lies in its contribution to optimizing patient safety, particularly in high-risk infant cases, making it essential reading for healthcare providers dealing with airway management challenges.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
The present article makes a noteworthy addition to the research on cranial nerve schwannomatosis. It diligently investigates a range of cases, providing valuable insights into the complexities and diverse presentations of the condition, especially when the vagus nerve is affected. The article distinguishes itself by conducting a comparative analysis and integrating a unique case related to nasopharyngeal carcinoma, thereby enhancing our comprehension of the clinical diversity associated with schwannomatosis. This article exemplifies a high level of scholarly dedication and expertise in its execution. It merits being considered for publication, provided that specific aspects are addressed to further enhance its academic significance.
The article makes a notable contribution to medical literature by providing an in-depth review of cranial nerve schwannomatosis, a less common form of neurofibromatosis. It examines 15 individual cases, with one case receiving extensive examination in a detailed case report. This analysis illuminates key aspects such as the specific cranial nerves affected, the age at which diagnosis typically occurs, and the current diagnostic criteria for cranial nerve schwannomatosis. The paper highlights the critical need to differentiate this condition from neurofibromatosis type 2, especially in patients with numerous schwannomas or significant nerve involvement. This differentiation is vital for effective clinical management, ongoing monitoring, and genetic counseling. While the paper is structured effectively and based on robust research methods, it might need some revisions before it can be considered ready for publication.
This article is a pioneering literature review on cranial nerve schwannomatosis, a rare neurofibromatosis subtype. Analyzing 15 cases, it finds schwannomatosis mainly affects the trigeminal and vagus nerves, with diagnosis ages ranging widely. It underscores differentiating schwannomatosis from neurofibromatosis type 2, stressing its consideration in patients with multiple schwannomas or extensive nerve involvement. The inclusion of a case report of a 58-year-old Chinese female adds practical relevance and aids in understanding the disease's clinical manifestation. This well-structured, methodologically sound paper significantly enhances clinical awareness and patient care in schwannomatosis. The article is recommended for acceptance; however, some issues require clarification to strengthen its contributions further.
Lee JYY, Logaswari M, Tan CS, Loh JMR, Tan LTH, Huang XY. Cranial nerve schwannomatosis: A case report and comprehensive literature review on a distinct variant of neurofibromatosis. Arch Otorhinolaryngol Head Neck Surg 2024;8(1):4. https://doi.org/10.24983/scitemed.aohns.2024.00184