As procedures become increasingly complex, the current surgical techniques become increasingly strained with the demand for a new idea bringing about the birth of some new concept or technique. Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, we take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
In order to enable the surgical procedure to move from a macroscopic to a microscopic one, the use of fine instruments is necessary. Traditionally, three main instruments are needed, including a microscope, fine operating instruments, and fine suture material. Although suturing techniques prevail in both experimental and clinical settings, considerable interest exists in the alternative mechanical techniques. Our review focuses on the newly emerging sutureless microvascular anastomosis techniques [1,2].
Several disadvantages to the sutured anastomoses have been identified. Some of these are related to the material itself and others are due to the process. The projection of potentially prothrombotic suture material into the vessel lumen and myointimal hyperplasia secondary to foreign body reaction in the wall of the blood vessel are the disadvantages due to the material itself [3]. The process of pure manual maneuver also has its disadvantages, which include the potential to cause damage to the vessels and a prolonged operating time. Ever since the 1900’s, research had started on other methods of vascular anastomosis and still being continued till this day, concerning the use of lasers, tissue adhesives, extraluminal cuffing rings, and everting pinned-ring devices, metallic stapling devices, and magnets.
A short history of these devices shows the continuous quest for innovative equipment and the increased performance. Lasers have been used for vessel welding since 1979. Jain and Gorisch were the first to report on vascular anastomoses using a neodymium: yttrium- aluminium-garnet laser. Vessel ring anastomosis commenced with Payr’s design (1904) of interlocking magnesium rings, similar to Henroz’s device of bowel anastomosis. Small pins on one side kept the vessel ends everted. The pins passed full-thickness through both vessel walls and the holes in the matching ring before being bent to secure the anastomosis. In 1913, Landon developed a metal ring that was smooth on one end and contained 5 slightly everted teeth on the other. In 1962, Nakayama simplified the initial ring stapler device into two rings that were joined onto each other through 2 flanges, one with 6 pins and the other with 6 holes, to receive the pins of the opposite flange. In 1986, Ostrup and Berggren presented the Unilink System, improving the Nakayama design. It consisted of two polyethylene rings with alternating stainless-steel pins and holes. The most commonly used device was the Coupler from Synovis. In 1992, Kirsch proposed the microvascular anastomosis based on the principle of flanged, extra-vascular, intimal approximation by arcuate-ledges stainless steel clips. Vessel ends underwent 90 degrees eversion and were then held together with extravascular staples. Magnetic energy was introduced in vascular surgery by Obora in 1978 using magnetic rings and hollow cogwheel-shaped metal devices held together by magnetic energy to anastomose vessels up to 2 mm [1,2].
The advantages of these sutureless devices are many, including good contact between the intima, higher patency rates (because there was no exposure of anastomotic material into the lumen), shorter operating times, and less need for microsurgical training, making it increasingly feasible for the less experienced surgeon. Some devices, because they are simple, efficacious, and significantly faster than suturing material, are, in some units, the preferred method of microvascular anastomosis. The best example would be the Coupler for venous anastomosis.
Although the above would be the principles upon which all of the sutureless devices would be based on, they also had some generic disadvantages: sometimes complex and cumbersome instrumentation, reduced vessels distensibility (a rigid foreign body enclosed a dynamic dilating structure), not applicable for significant vessel size discrepancies or end-to-side anastomoses, and increased vessel consumption into the device, which would be contraindicated in growing children because they would not allow for increases in vessel diameter. All the specific advantages and disadvantages of the techniques are discussed in the review article.
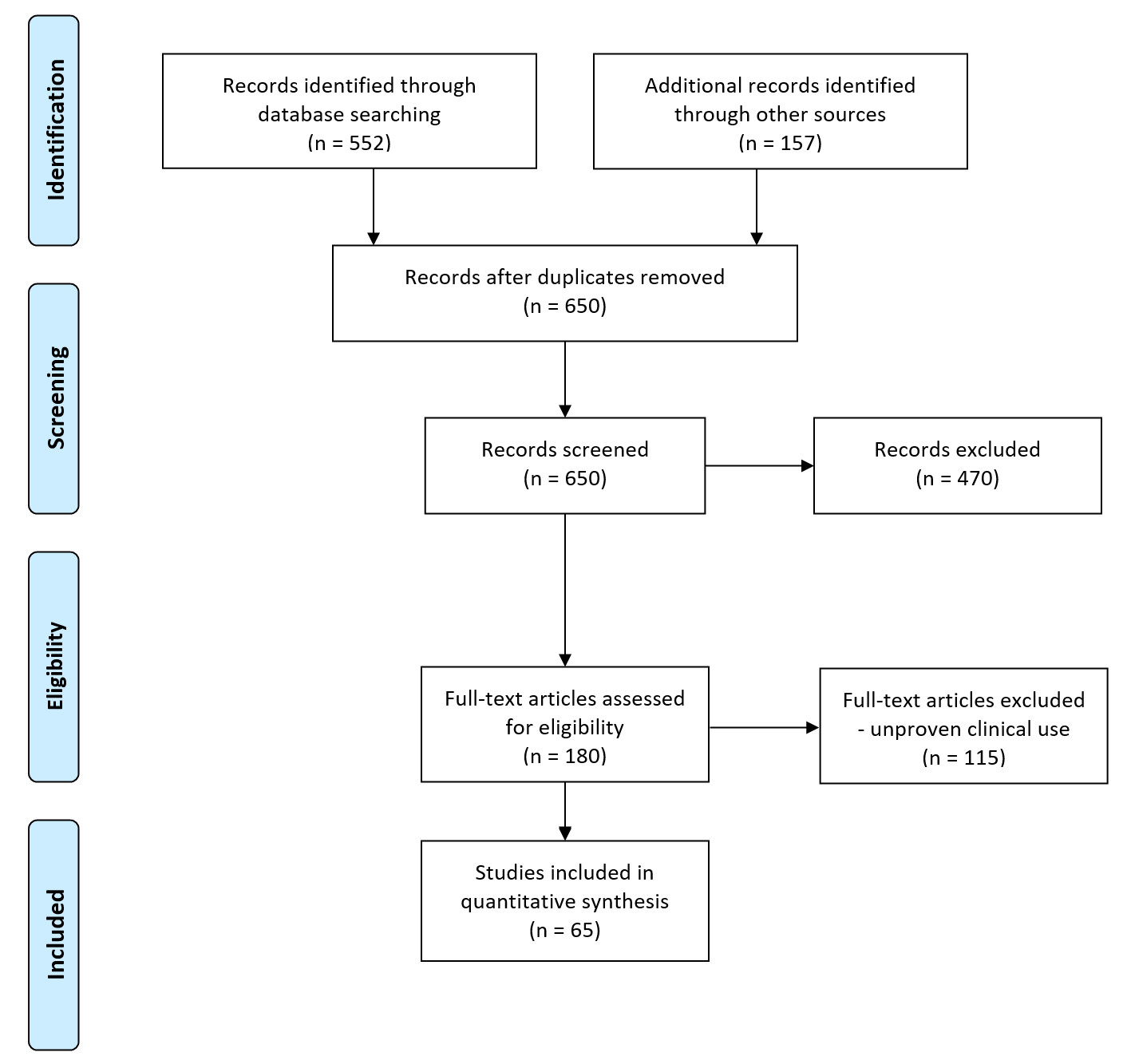
This study followed the methods used in Preferred Reporting Items for Systematic Reviews and Meta-Analyses [4]. Eligibility criteria were specified unambiguously to ensure that studies were selected in a systematic and unbiased manner. Via PubMed, a Medline literature search was performed. Keywords used were sutureless microvascular anastomosis, vascular closure staples, laser, tissue adhesives, and Unilink. This was combined with cross-referencing from the reference lists of all recovered and relevant publications on this subject. In total, 650 publications were discovered, of which, 180 were relevant. The inclusion criteria were sutureless devices or sutureless procedures for microvascular anastomosis, English language, clinical studies, and animal research on technologies already used in clinical practice or on refinements of existing techniques. Out of the 470 articles excluded, 120 were non-animal studies or animal experimental studies with low numbers, and 350 articles were not relevant to our topic (Figure 1).
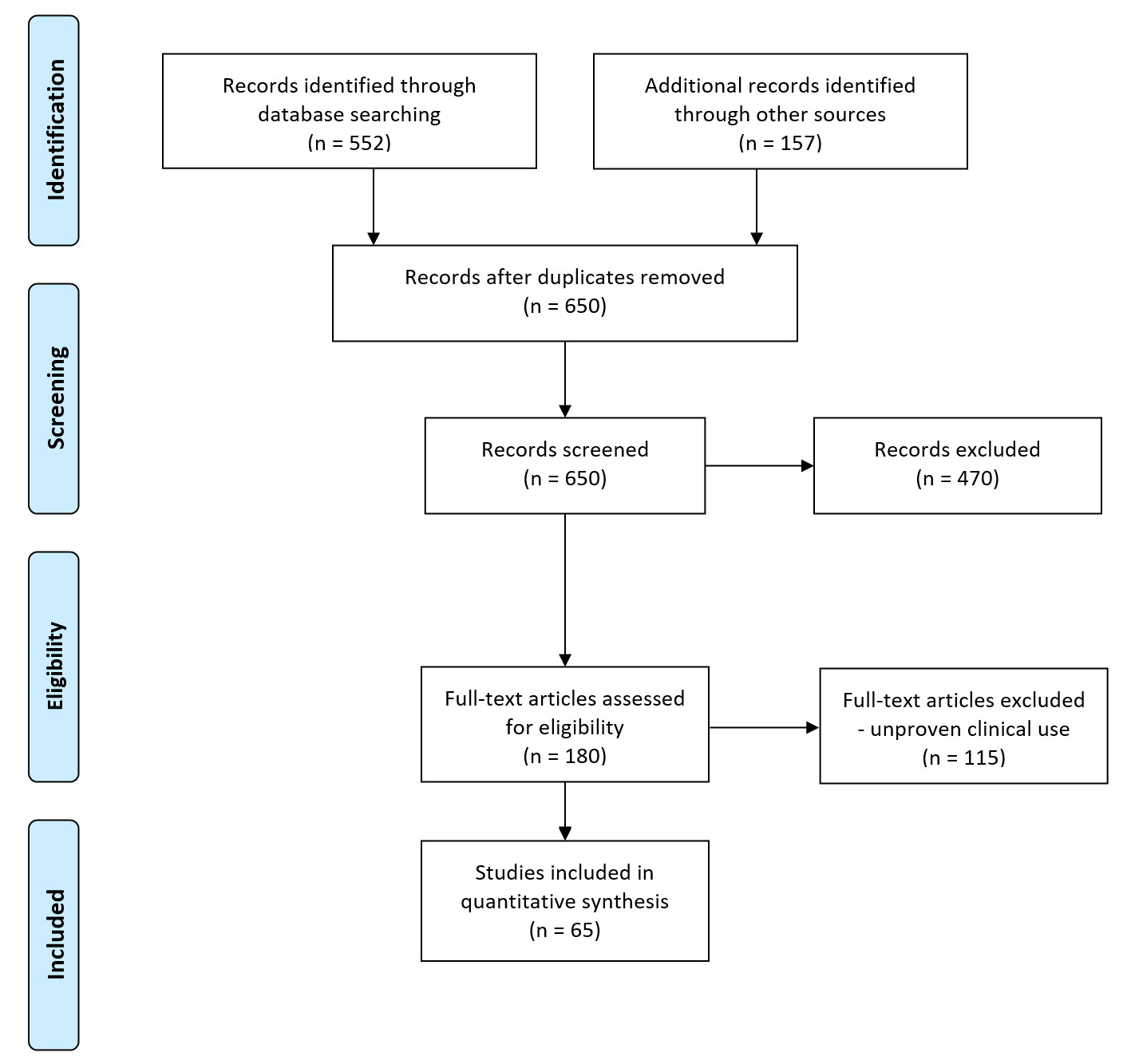
Figure 1. Flow diagram of study retrieval and selection.
In tissue, laser light can be reflected, absorbed, scattered, or transmitted, the absorption being the most important interaction in laser-Assisted Vascular Anastomosis (LAVA). The resultant energy is used to weld the edges of the vessel together. Laser power (watts) and the amount of energy and time required vary for the types of laser and for the size of the vessels. When the carbon-dioxide laser is used, the tissue temperature rises to 80-120 degrees Celsius and adhesion occurs through melting of collagen and coagulation of cells in the media and adventitia. In wound healing, this coagulum is gradually replaced by fibrous and muscular tissue. Argon lasers generate a surface temperature of 43-48 degrees Celsius, which is below the temperature at which collagen degenerates. In this case, the protein bonds are degraded thermally, allowing proteins to rebind to adjacent proteins in a smooth tissue-tissue connection.
The balance between over- and under-exposure of tissue is critical. At a given wavelength, the absorption of laser energy varies within different tissues. Each tissue has its own wavelength-specific absorption coefficient. Tissue absorption, scattering, and the predominant direction of scattering determine the penetration depth of a laser. In the case of moderate to high tissue absorption, it is likely that the penetration should optimally be limited to the adventitia to prevent reactive intimal hyperplasia. In larger vessels, the arterial wall or adventitia layer may be thicker than in microvessels, and thus deeper penetration of laser light and heat is allowed and required. The deposited energy then converts into heat, resulting in a local temperature increase that is responsible for the tissue alterations. The laser parameters important in determining the interaction with tissue are wavelength, power, power density (the intensity of the laser light in terms of power in Watts per unit area) and/or spot size, and time of exposure. The lasers used for LAVA are the carbon-dioxide laser, the Neodymium: Yttrium-Aluminum-Garnet laser, the diode laser, and the argon laser [5].
The lasers have been used in combination with different kinds of protein solutions used as solders and/or dyes in order to create an anastomosis of sufficient strength to withstand physiologic variations in blood pressure. Solders have greatly contributed in optimising LAVA by increasing the leaking point pressure (the pressure at which leakage from the anastomosis is seen). They consist of a protein or protein-like substance applied to the weld to enhance bonding by enlarging the bonding surface. Solder also acts as a heat sink to reduce thermal damage. By adding a wavelength-specific chromophore or dye to the welding site or solder, the laser absorption is greatly enhanced, allowing for more selective heating of tissue with less injury to underlying tissues [6-15].
We identified four clinical series using 1950-nm diode laser [16-19], which were performed after extensive animal experience [20]. This laser seems to have an ideal wavelength for microvascular anastomoses with a 150 mm penetration that matches the thickness of the vascular wall’s adventitia and media [21]. This allows for welding of the vessels without the use of a chromophore or solder preparation, with minimal aneurysm rates and better flow when compared with traditional procedures on MRI [22].
The advantages of LAVA include good patency rates, shorter operating time, reduced needle trauma, and less foreign body reaction. Unlike other sutureless techniques, the diameter mismatch and toxic reactions are no issue in LAVA. Coaptation of vessel walls, however, is important. Ideally, there should be perfect alignment of intima against intima without bulging or wrinkling. This technique can be used in both arterial and venous anastomosis.
As with every other technique, LAVA does also have its disadvantage. Thermal injury can lead to pseudoaneurysm formation. This is one of the reasons for stay stitches still being used. There are several other causes for aneurysms postulated in literature, such as loss of elastic lamina, inappropriate laser parameters, poor apposition of the vessel edges, and inadequate tensile and bursting strength [23-25]. The best way to prevent aneurysm formation is to minimize anastomotic disruption, which is achieved by careful vessel approximation and use of solders [26].
The fibrin glue mimics the final steps of the coagulation cascade to produce a physiologic fibrin clot. It was first used in microvascular anastomosis by Matras et al. and Pearl et al in 1977. Several studies have already reported the utilization of fibrin glue in microvascular anastomoses to complement the LAVA, increasing the bursting strength and decreasing the aneurysm rate [27], or to minimize the number of sutures and to decrease the operative time in conventional suture anastomosis. The technique of clotting fibrinogen solution around the anastomosis does not cause an increase in the rate of thrombosis, but actually strengthens the anastomosis [28]. In one study, the application of fibrin glue decreased the number of sutures required to complete the arterial and venous anastomoses down to 40%, while maintaining adequate patency rates and mechanical strength. Anastomoses were easier and faster to perform [29]. This proved a very useful adjunct in digit replantation, with at least two anastomoses per digit and possibly more than 20 needed in the case of multiple digit replantations requiring vein grafts. The time saved by using fibrin glue can be substantial [30].
Advantages
Overall, fibrin glue was found to decrease the number of sutures and operating time with the same immediate and late patency rates. It is safe and reliable, with no secondary effects, the histological analysis showing no significant differences compared to suturing only [31].
Disadvantages
Sagi et al. used a combination of Vicryl rings and fibrin glue for microvascular anastomosis [32]. They reported an insufficient bonding force for this glue to hold the cut ends of vessels together. Furthermore, some studies suggested that the fibrin glue may increase thrombogenicity when applied onto a vessel. This was refuted by Drake et al. and Frost-Arner et al. who demonstrated that the application of lower concentrations (500 IU/ml) of thrombin in conjunction with fibrinogen did not increase thrombogenicity in epigastric free flap models. The glue takes more time and is more complex to prepare. There is also a theoretical risk of viral disease transmission [32-34].
The venous coupler ring-pin system was first described in the literature by Nakayama in 1962. The Unilink system (3M) based on the same concept, now known as the Microvascular Anastomotic Coupler System (Synovis Micro Companies Alliance, Birmingham, AL), was described by Berggren and Ostrup in 1987.
The Unilink system consists of polyethylene rings with six alternating stainless-steel pins and holes in each ring and an apparatus to approximate the rings and cut vessel ends. Vessel ends are passed through opposing rings and everted on the pins; the coupler device approximates the rings, which stay in place. Studies have evaluated the success of this technique in joining small arteries and veins by using histologic and scanning electron microscopy and also by comparing the patency rate of anastomoses and flap survival with standard suture technique [35,36]. In some units, the coupler device is the preferred method for performing the venous microvascular anastomosis in free tissue transfers [37].
Anastomotic COUPLER System (Synovis) has been specifically designed for use in the anastomosis of veins and arteries having an outside diameter not less than 0.8 mm and not larger than 4.3 mm, and a wall thickness of 0.5 mm or less.
The hemodynamic consequences of anastomosing small arteries and veins with this device are minimal and the hemodynamic characteristics of the repaired vessels recover with the course of the normal healing process. The mean anastomosis time cited in the literature for the artery procedures was 8 minutes and for vein procedures 10 minutes.
Because it is a rigid structure that stretches the friable small vessels between it and the vascular clamps, the main concern would be the histological changes that occur in the vessel wall. The media remains viable outside the device but undergoes patchy necrosis within the device. Intimal hyperplasia occurs adjacent to and within the device. A circumferential triangular zone with loose connective and vascular channels forms at the component junction in 3 weeks. Evidence of injury from both clamp application and the strain of approximation are occasionally present [36-39].
Overall, many of the histopathologic features of the polyethylene ring-pin device are, in general, similar to those observed in suture anastomoses. These features include early sloughing of the endothelium, re-endothelialisation by 3 weeks, early intimal and medial inflammatory processes, resolution of inflammation within 3 weeks, disruption of the internal elastic lamina with a slow process of restoration, medial necrosis near suture or device materials, and local giant cell responses [36,40,41].
Apart from the decreased anastomotic time, which is one of the main advantages of this device, a 20 MHz ultrasonic Doppler has been specifically designed for use in end-to-end anastomosis for the detection of blood flow, in order to confirm vessel patency intra- and postoperatively at the anastomotic site.
In the literature, the incidence of thrombosis in a hand-sewn venous anastomosis is as high as 10%, especially in lower limb trauma reconstruction, and less than 3% in breast reconstruction. The systematic review by Ardehali et al 2014 demonstrates the thrombosis rate with the coupler range from 0 to 3% and less variation compared to the hand-sewn technique. No case-control prospective study or a randomised control trial was identified in the database search [42-47].
With the use of the device over the last twenty years, two main drawbacks have been pointed out. First, a ring-pin device has metallic pins on its ring that penetrate the vessel wall from the outside and permanently remain inside the vessel walls. Although the metallic pins allow very good fixation to the vessel walls, the pins can interfere with the normal restoration and the remodelling process after installation. The vessel walls are atrophied because of the continuous pressure of the blood flow against the rigidly fixed non-absorbable ring-pins complex [36,48,49]. Second, although the procedure for mounting the vessels onto the device is much faster than suturing the vessel walls, surgeons still must manually insert the metallic pins into the vessel walls.
Also, surgeons prefer to use the device for venous anastomosis only. Arterial wall is too thick and stiff most of the times. Although described, the end-to-side anastomosis with the coupler is rarely performed.
The vacuum-assisted microvascular anasto coupler (VaMAC) presents a dramatic change in the principle and structure of the device that solves these two problems. The VaMAC uses negative pressure as an atraumatic force to fix vessel walls instead of traumatic metallic pins. Negative pressure is also very versatile because surgeons only have to place the vessel walls in the appropriate positions of the device, and the device may semiautomatically attach the vessel walls to the device. The VaMAC was designed to eliminate the need for metallic pins and to shorten the time of the procedure and was tested in rats [49]. This device has not yet been used in clinical practice.
The vascular closure staples (VCS) clip clinches to the vessel wall, everting but not penetrating the endothelium, yet grasping the adventitia firmly without crushing. Eversion-stapled anastomoses appear to be more reliable because of the absence of intraluminal foreign bodies, permanent endothelial continuity, avoidance of intimal or mucosal penetration, avoidance of platelet aggregation, and effective re-endothelialization before day 7 [50,51].
VCS clips represent a major advance in the growing-vessel surgery. They allow vessel growth (as do interrupted sutures) and enable enhanced healing owing to the absence of foreign thrombogenic and hyperplasic material on the intimal surface [52].
In 2002, Zeebregts et al. published a comparative study of different techniques used to create microvascular anastomoses in free-flap reconstructions. They performed 474 microvascular anastomoses in 216 consecutive free-tissue transfers. The anastomosis techniques included manual sutures, Unilink rings, and VCS clips. The mean anastomotic time when rings were applied was significantly shorter than when using clips or sutures. Venous anastomoses using clips took less time than those using sutures. Early flap failure was caused by the failure of the arterial anastomosis in eight cases; all of them were sutured. None of the clipped arterial anastomoses failed. Three of the early flap failures due to the failure of venous anastomosis were sutured, seven were anastomosed with rings, and one was clipped. Both the VCS clip-applier system and the Unilink system are easy to handle and allow fast microvascular anastomoses without intraluminal penetration. The patency rate of clipped vessels is at least as good as the patency rates of vessels anastomosed using sutures or rings [53].
The major pitfall of VCS is the deceptively easy application of clips from the anastomotic applier. Although the learning curve for clip application is steep, there is the real and fundamental need for symmetrical vessel wall eversion and approximation with additional corner stitches and the use of everting forceps prior to clip placement. The demand for symmetrical eversion prior to application of a secure clip requires skill and practice, but not to the extent of suture anastomosis [54]. Vascular clips have no significant effect on cellular proliferation, intimal/media changes, or peak systolic velocity at anastomoses [55-57].
Use of tissue adhesives started from the same search of an easy-to-apply and time-saving technique for microvascular anastomoses. They do, however, necessitate the use of additional sutures in order to prevent aneurysm formation. The prime concern with glues is that they can give rise to allergic reactions and anaphylaxis.
Experimental end-to-end anastomoses have been performed successfully using bucrylate glue (isobutyl-2-cyanocrylate) and histoacryl glue (butyl-2-cyanoacrylate) with two or three stay sutures. The use of Histoacryl glue showed minimal toxicity and was comparable to suture anastomosis. The glued anastomosis was also associated with a shorter completion time, less bleeding, and equivalent patency; the sutureless anastomosis was associated with less intimal thickening compared with the traditional suture group. However, problems were reported with distortion of the vessels by hardened glue and/or thromboses secondary to glue entering the lumen. To overcome these imperfections, a new technique using Histoacryl glue with an intravascular soluble stent to keep the lumen widely patent and prevent intrusion of glue into the lumen was developed [58,59]. The technique was found to be efficient, fast, easy to learn, and readily accessible to the minimally experienced surgeon. No bleeding at the time of clamp removal was observed. However, the pathological study revealed histotoxicity of the glue on the arterial wall without consequence on efficacy [60].
Histological analysis of anastomoses done by suture or by application of bucrylate (isobutyl- 2-cyanoacrylate) following placement of three stay sutures showed degeneration of the media and deposition of calcium in the vessels in the bucrylate group. In addition, a more intense and prolonged foreign-body giant-cell response was noted in comparison to the vessels in the suture group [58].
Following the stent principle, Gurtner used a synthetic polymer - poloxamer assisted anastomoses. When the blood vessels were filled, the liquid polymer was heated. The liquid phased into the solid state at 38 degrees Celsius and was solid at 40 degrees Celsius. The floppy vessels stiffened like straws and could be lined up end-to-end. The vessels were sealed together with Dermabond, and then the clamps were released. The normal body temperature blood rushed into the vessels and hit the thermoreversible polymer. The polymer dissolved within one to two seconds and was excreted by the kidneys. There was no evidence of embolization, toxicity, or end-organ damage. Sutureless anastomoses were completed more efficiently than hand-sewn anastomoses with equivalent patency and burst strengths. Inflammation and scarring were dramatically decreased in the sutureless group. Dermabond, a new class of cyanoacrylate, 2-octyl cyanoacrylate, had a longer chain and was less histotoxic than other tissue adhesives [61].
The research done up to now on vascular anastomoses with the use of magnets was mainly on large animal models (foxhound femoral artery) and as side-to-side anastomoses. One longitudinal arteriotomy was performed distal to the proximal clamp and another proximal to the distal clamp. A deployment device firmly held one magnet (with an oval lumen) at the tip of the device and another identical magnet several millimetres above the first with the two magnets aligned. The magnet at the tip was then inserted through the arteriotomy into the proximal artery. When the intravascular magnet was centred in the arteriotomy with slight traction, the deployment device trigger was activated; the magnets attracted and compressed the arterial wall forming a two-magnet vascular port. An identical two-magnet port was created near the distal end of the occluded arterial segment [62-64].
As the current surgical practices are being challenged with increasingly intricate operations, the demand on microsurgery has, and can only be expected, to increase. While the traditional microscope and fine suture have proven to be an invaluable source of healing for many patients, it is still nevertheless labour and time intensive, and not without its complications. From more than a century ago, thought and effort had gone into the development of sutureless microvascular anastomoses, with the aim of increasing the success of the procedure, reducing operation time and reducing the need for subspecialist training, thereby making it increasingly available to the patient population that would otherwise benefit from it. Laser-assisted vascular anastomoses, tissue adhesives, and magnets are all promising ideas, with progress in this field leading to improved anastomotic times and ultimately to better patient outcomes as demonstrated by the coupler system and vascular closure staples.
Received date: July 08, 2018
Accepted date: October 04, 2018
Published date: April 25, 2019
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
© 2019 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
A novel technique of sequential ETS micro-venous anastomoses using three vessel loops for IJV occlusion and a single vascular clamp to retract and hold the anastomoses sites in position.
The authors present a novel synthetic vascular model for microanastomosis training. This model is suitable for trainees with intermediate level of microsurgical skills, and useful as a bridging model between simple suturing exercise and in vivo rat vessel anastomosis during pre-clinical training.
Improving efficiency of microvascular anastomosis by combination of open-loop and airborne suture techniques.
Authors report a case of lower extremity lymphedema treated by LVA that preoperatively mapped not only lymphatic vessels by PDE, but also veins and venules using Veinsite™ .
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
Optimum operating conditions are an integral part of a successful microvascular surgery. We hereby introduce a new technique to make a low- cost microsuction cannula, which will be helpful for microvascular surgeons.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
Authors proposed a simple innovative technique that helps in achieving a clear surgical field at the time of venous repair by diverting the venous bleeding through a glove finger and at the same time preventing venous congestion of the flap.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
This article presents a case study on the successful replantation of a pediatric lower lip after a dog bite, focusing on artery-only microanastomosis. It highlights the challenges and effective strategies in pediatric microvascular surgery, particularly emphasizing the importance of specialized surgical techniques and thorough postoperative care. Alongside the case study, an extensive literature review supports the feasibility of artery-only anastomosis and the traditional yet critical use of leech therapy for managing venous congestion. This research is vital for medical professionals specializing in pediatric surgery, offering key insights into improving both functional and aesthetic outcomes for young patients. Additionally, it identifies gaps in long-term research and stresses the need for ongoing studies to refine treatment protocols, making it an indispensable resource for enhancing patient care and outcomes in pediatric reconstructive surgeries.
The PLOSEA technique detailed in this study addresses the significant challenge of managing large vessel size discrepancies in microvascular surgery with an innovative and accessible method. By partially obliterating the larger vessel lumen before anastomosis, the technique reduces risks of thrombosis and misalignment, simplifying the procedure without sacrificing effectiveness. This advancement is particularly valuable as it allows surgeons with varying levels of experience to perform complex reconstructions with greater confidence and improved patient outcomes. A key feature is the inclusion of a detailed video demonstration, providing a dynamic and comprehensive visual guide that surpasses traditional static images. This video meticulously elucidates each procedural step, enhancing understanding and facilitating the practical application of the technique. Emphasizing technical precision, patient safety, and surgical efficiency, this study offers a compelling narrative for medical professionals. The transformative impact of the PLOSEA technique on surgical practice underscores its importance, presenting a novel approach that can enhance the quality of care and expand the capabilities of microsurgeons worldwide.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
A novel technique of sequential ETS micro-venous anastomoses using three vessel loops for IJV occlusion and a single vascular clamp to retract and hold the anastomoses sites in position.
The authors present a novel synthetic vascular model for microanastomosis training. This model is suitable for trainees with intermediate level of microsurgical skills, and useful as a bridging model between simple suturing exercise and in vivo rat vessel anastomosis during pre-clinical training.
Vessel-compromised neck is a challenge for the microvascular surgeon planning for a free tissue transfer. This study describes the authors’ experience regarding a successful free tissue transfer in a vessel-compromised neck. The authors also propose an algorithm for management of vessel-compromised neck.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
This article presents a case study on the successful replantation of a pediatric lower lip after a dog bite, focusing on artery-only microanastomosis. It highlights the challenges and effective strategies in pediatric microvascular surgery, particularly emphasizing the importance of specialized surgical techniques and thorough postoperative care. Alongside the case study, an extensive literature review supports the feasibility of artery-only anastomosis and the traditional yet critical use of leech therapy for managing venous congestion. This research is vital for medical professionals specializing in pediatric surgery, offering key insights into improving both functional and aesthetic outcomes for young patients. Additionally, it identifies gaps in long-term research and stresses the need for ongoing studies to refine treatment protocols, making it an indispensable resource for enhancing patient care and outcomes in pediatric reconstructive surgeries.
The PLOSEA technique detailed in this study addresses the significant challenge of managing large vessel size discrepancies in microvascular surgery with an innovative and accessible method. By partially obliterating the larger vessel lumen before anastomosis, the technique reduces risks of thrombosis and misalignment, simplifying the procedure without sacrificing effectiveness. This advancement is particularly valuable as it allows surgeons with varying levels of experience to perform complex reconstructions with greater confidence and improved patient outcomes. A key feature is the inclusion of a detailed video demonstration, providing a dynamic and comprehensive visual guide that surpasses traditional static images. This video meticulously elucidates each procedural step, enhancing understanding and facilitating the practical application of the technique. Emphasizing technical precision, patient safety, and surgical efficiency, this study offers a compelling narrative for medical professionals. The transformative impact of the PLOSEA technique on surgical practice underscores its importance, presenting a novel approach that can enhance the quality of care and expand the capabilities of microsurgeons worldwide.
The clinical significance of otitis media with effusion (OME), a complication associated with cleft lip/palate (CLP), is often overlooked in children. The author reviews the pathogenesis, clinical manifestations, and diagnoses of OME in children with CLP as well as the controversies surrounding treatment. He also provides a flowchart to guide the management of OME in children with CLP.
Epistaxis (i.e., nosebleed) is a common otolaryngologic emergency; however, it is seldom life-threatening and most minor nosebleeds stop on their own or under primary care from medical staff. Nonetheless, cases of recurrent epistaxis should be checked by an otolaryngologist, and severe nosebleeds should be referred to the emergency department to avoid adverse consequences, including hypovolemic shock or death. This paper reviews current advances in our understanding of epistaxis as well as updated treatment algorithms to assist clinicians in optimizing outcomes.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
This article presents a comprehensive review of schwannomatosis affecting cranial nerves, delineating its unique characteristics distinct from other forms of neurofibromatosis. By addressing diagnostic complexities and the evolving criteria for identification, the paper emphasizes the critical need for accurate recognition of schwannomatosis to facilitate effective management and provide essential genetic counseling. Enriched with a detailed case study, this review delivers vital insights into the epidemiology, symptomatology, and therapeutic strategies for schwannomatosis, advocating for a revision in current clinical approaches. This work is indispensable for medical professionals aiming to enhance diagnostic precision, comprehend genetic underpinnings, and improve patient outcomes. Offering a thorough analysis of this rare condition, the article is pivotal not only for clinicians and researchers in the neurogenetic field but also for a broader spectrum of medical and scientific communities, bridging a notable gap in contemporary medical literature.
The author reframes review articles as evidence infrastructure that updates clinical consensus and identifies research gaps. The author argues that narrative reviews integrate findings across biological scales and translate molecular data into clinically meaningful explanations of disease behavior. Using cholesteatoma, the author illustrates how molecular evidence can recast invasiveness and recurrence into a mechanistic model that also motivates the pursuit of nonsurgical therapies. The author contrasts this interpretive role with systematic reviews, which function as reproducible decision chains grounded in transparent, verifiable methodology. Finally, the author cautions that AI assisted automation can propagate error unless it is governed by expert oversight within a human in the loop model that preserves auditable decision chains.
Ilie V, Haddad R, Moisidis E. Sutureless microvascular anastomosis: Literature review. Int Microsurg J 2019;3(2):1. https://doi.org/10.24983/scitemed.imj.2019.00112