Objective: A novel surgical technique to reanimate a paralyzed face, utilizing the functional transplantation of the pronator quadratus muscle (PQM) in combination with a radial forearm flap (RFF) to cover a soft-tissue defect, was recently described. Although it is known that this muscle is supplied by the interosseous artery, a flap combining the radial artery has shown sufficient vascularization of this muscle as well. The PQM is generally poorly described in the literature, and there is insufficient information regarding its anatomical landmarks and vascular supply. The aim of this study was to specify the anatomical features of the PQM and provide a description of its vascularization by the radial artery using fresh cadavers.
Methods: The study involved the dissection of 20 pronator quadratus muscles from 10 fresh cadavers. To visualize the muscle branches within the PQM, the radial artery was injected with a methylene blue solution. Measurements were taken for the number of muscle branches, innervation, length, weight, and width of the PQM. Additionally, anatomic landmarks based on the radius bone were documented.
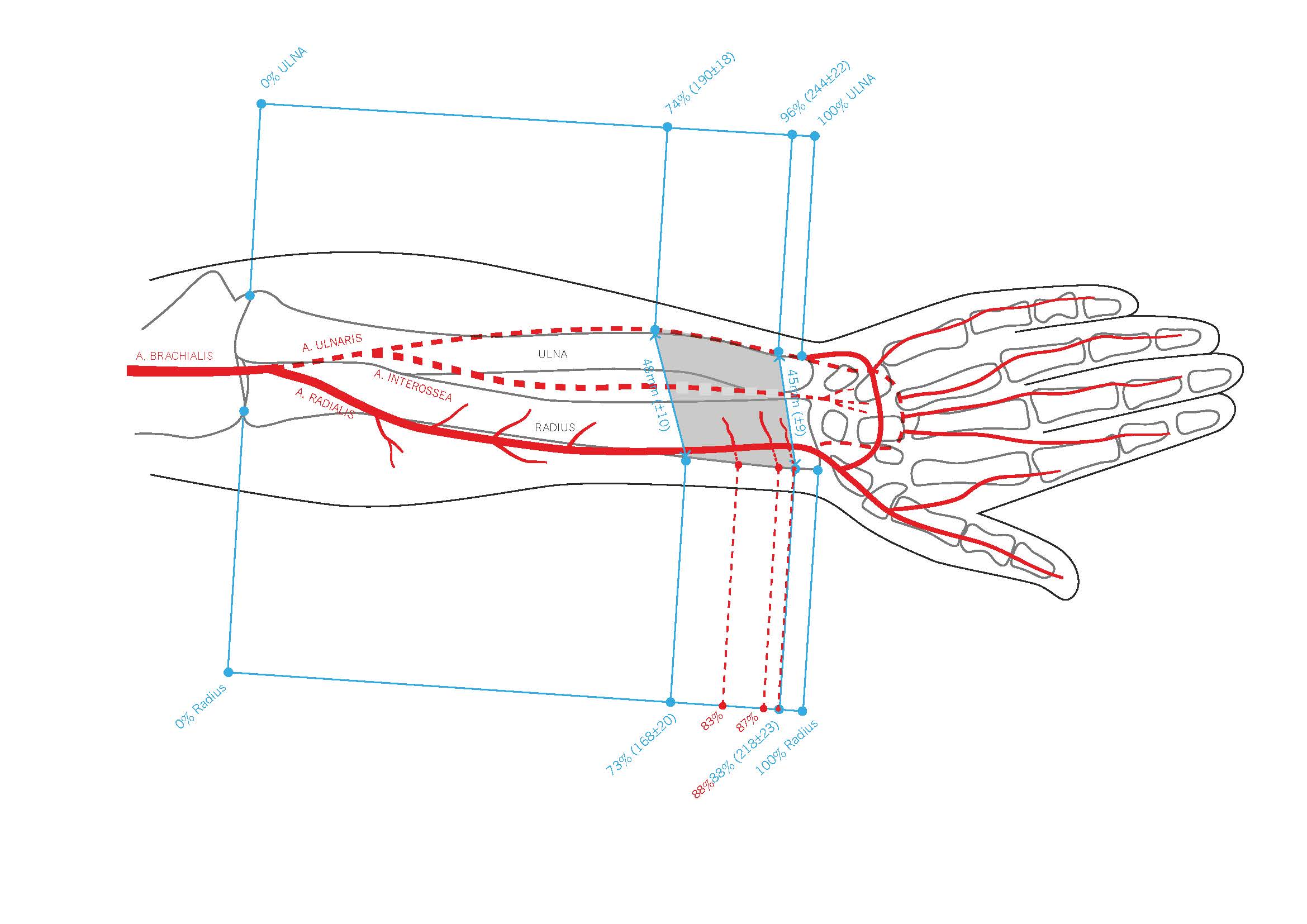
Results: There were up to four muscle branches of the radial artery supplying the PQM. The average muscle weight was 8 ± 3 g, with a mean length of 50 mm and a mean width of 45 mm distally and 48 mm proximally. On the radius, the proximal border of the PQM averaged 169 ± 21 mm from the radial head, corresponding to 73 ± 3% of the radial bone length, while the distal border was at an average of 218 ± 24 mm (95 ± 3%). On the ulnar aspect, the proximal and distal borders of the PQM measured on average at 190 ± 18 mm (74 ± 4%) from the olecranon and 244 ± 23 mm (96 ± 3%), respectively.
Conclusion: There are up to four muscle branches of the radial artery, each under 0.5 mm in diameter, that arise from the radial artery in the area of the PQM origin and insertion. These branches should provide a constant blood supply to the PQM when it is raised with a free radial forearm flap as a chimeric flap.
Facial paralysis patients experience facial asymmetry, impaired emotional expression, and difficulties with activities such as eating, drinking, speaking, and communicating [1]. Nowadays, functional reconstruction methods offer established techniques for reanimating the faces of patients with irreversible facial palsy [2–11]. In cases involving the simultaneous resection of skin, muscle, and the facial nerve, the challenge lies in restoring facial function while achieving skin closure. A case report has described the use of a chimeric radial forearm flap with innervated pronator quadratus muscle (PQM), achieving satisfactory results [12]. This surgical technique reanimated a partially paralyzed face by transplanting the PQM functionally in combination with a radial forearm flap (RFF) to cover the soft-tissue defect [12].
However, a detailed description of this method regarding the PQM's blood supply, when combined with the radial forearm flap, has not been previously reported. In this study, the authors aim to revisit the anatomical landmarks of this flap and provide a comprehensive examination of the vascular anatomy based on the radial forearm artery, which is used for free functioning muscle transfer in facial reanimation.
Twenty arms were meticulously dissected in ten fresh adult cadavers (seven males and three females) at the Center of Anatomy, Medical University of Vienna, Austria, to study the diameter, length, and caliber of the radial artery branches leading into the PQM and their relationship to bony anatomical landmarks. Additionally, the weight and size of the PQM were assessed.
The dissection of the PQM flap followed the standard procedure for the radial forearm flap. Care was taken during the dissection of the radial artery in the distal quarter along the radius. When all flexor tendons were retracted ulnarly, the arterial muscle branches became visible, originating from the radial artery and running in an ulnar direction into the PQM muscle. The interosseous nerve could be dissected at the proximal border of the PQM, as close to its origin as possible, and then resected around the midpoint of the radius. After exposing the PQM branches and the interosseous nerve, the PQM muscle was gently elevated from the ulna and radius using a raspatory. The PQM blood supply was examined under 3x loupe magnification after injecting a methylene blue solution (C.I.52015, LabChem Röttinger, Dinslaken, Germany) into the origin of the radial artery. All measurements were taken from proximal to distal. On the radial aspect, measurements started from the radial head, while on the ulnar aspect, measurements began from the olecranon.
This study was conducted in accordance with the local ethics regulations of the Center of Anatomy and Cell Biology, Medical University of Vienna, Austria.
The PQM is predominantly a trapezoid-shaped muscle, with an average proximal width of 48 ± 10 mm, a distal width of 45 ± 9 mm, and an overall length of 50 ± 9 mm. The muscle's weight varied from 3.2 g to 14.6 g.
Among the PQMs (n = 20), 40% exhibited equal proximal and distal widths, 50% had a wider proximal width than distal, and only 10% (n = 2) of all muscles were wider at the distal end than the proximal.
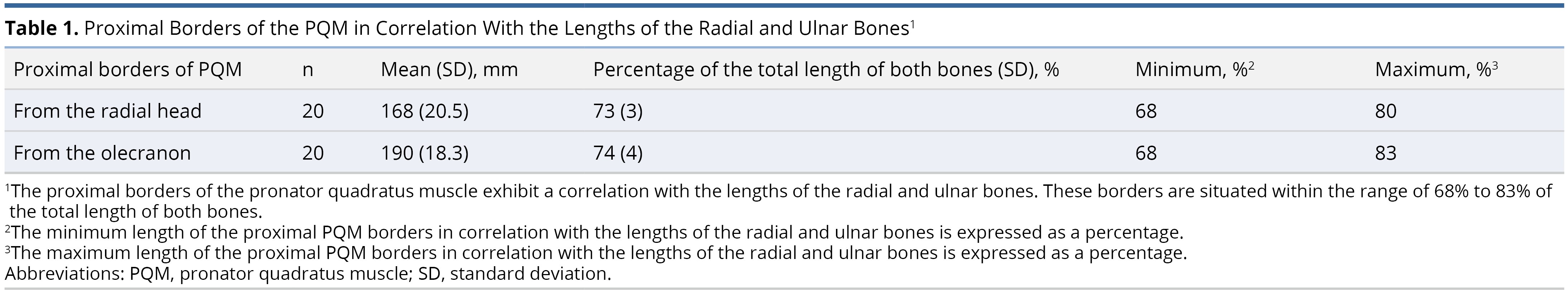
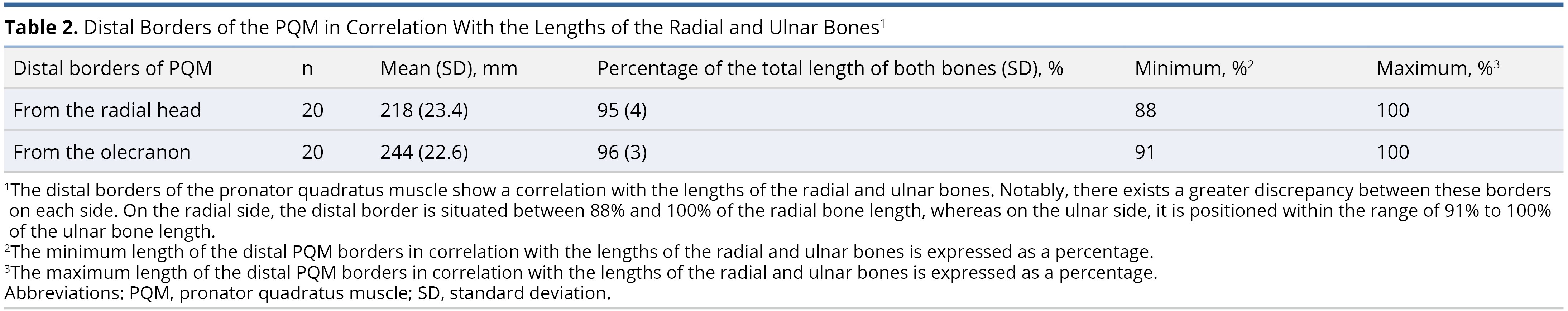
The radial bone had an average length of 230 ± 24 mm. On the radial side, the proximal borders of the PQM were found, on average, at 169 ± 21 mm from the radial head, accounting for 73 ± 3% of the radial bone's length, while the distal border was at 218 ± 24 mm (95 ± 3%).
On the ulnar aspect, the proximal and distal borders of the PQM were measured at 190 ± 18 mm (74 ± 4%) from the olecranon and 244 ± 23 mm (96 ± 3%), respectively. We did not differentiate between the superficial and deep heads of the muscle (Table 1 and Table 2).
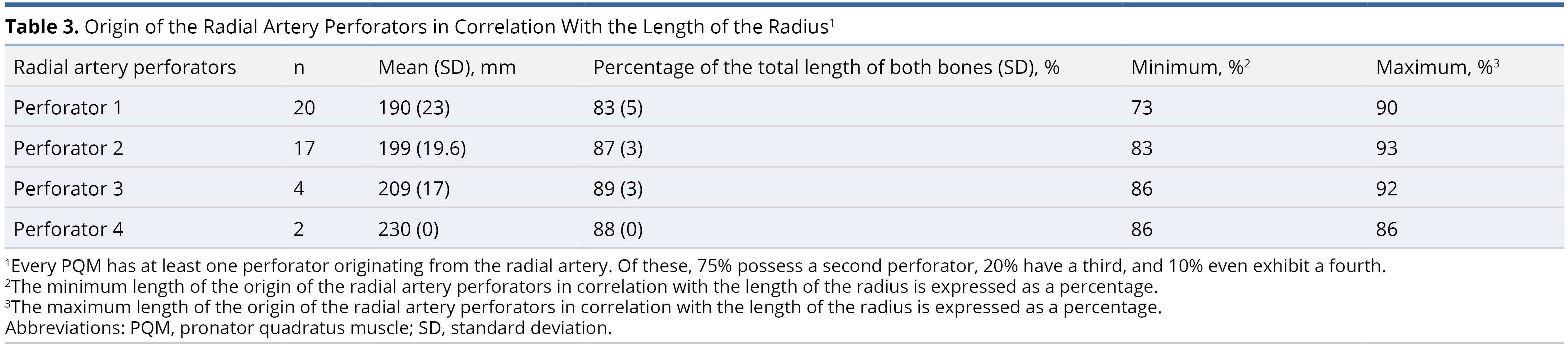
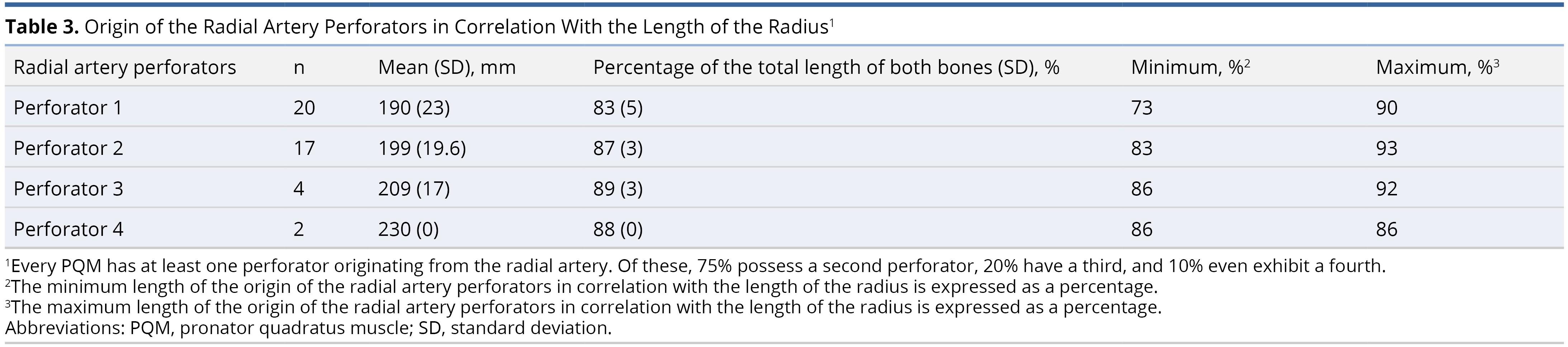
The average origin of the radial artery was 32 ± 9 mm from the proximal border of the head of the radius (caput radii). All PQMs had at least one muscle branch of the radial artery, which was found, on average, at 190 ± 23 mm (83%) from the radial head. Among the PQMs, 85% (17 out of 20) had a second muscle branch, 20% (4 out of 20) had a third branch, and 10% (2 out of 20) had a fourth branch (Table 3, Figure 1, and Figure 2).
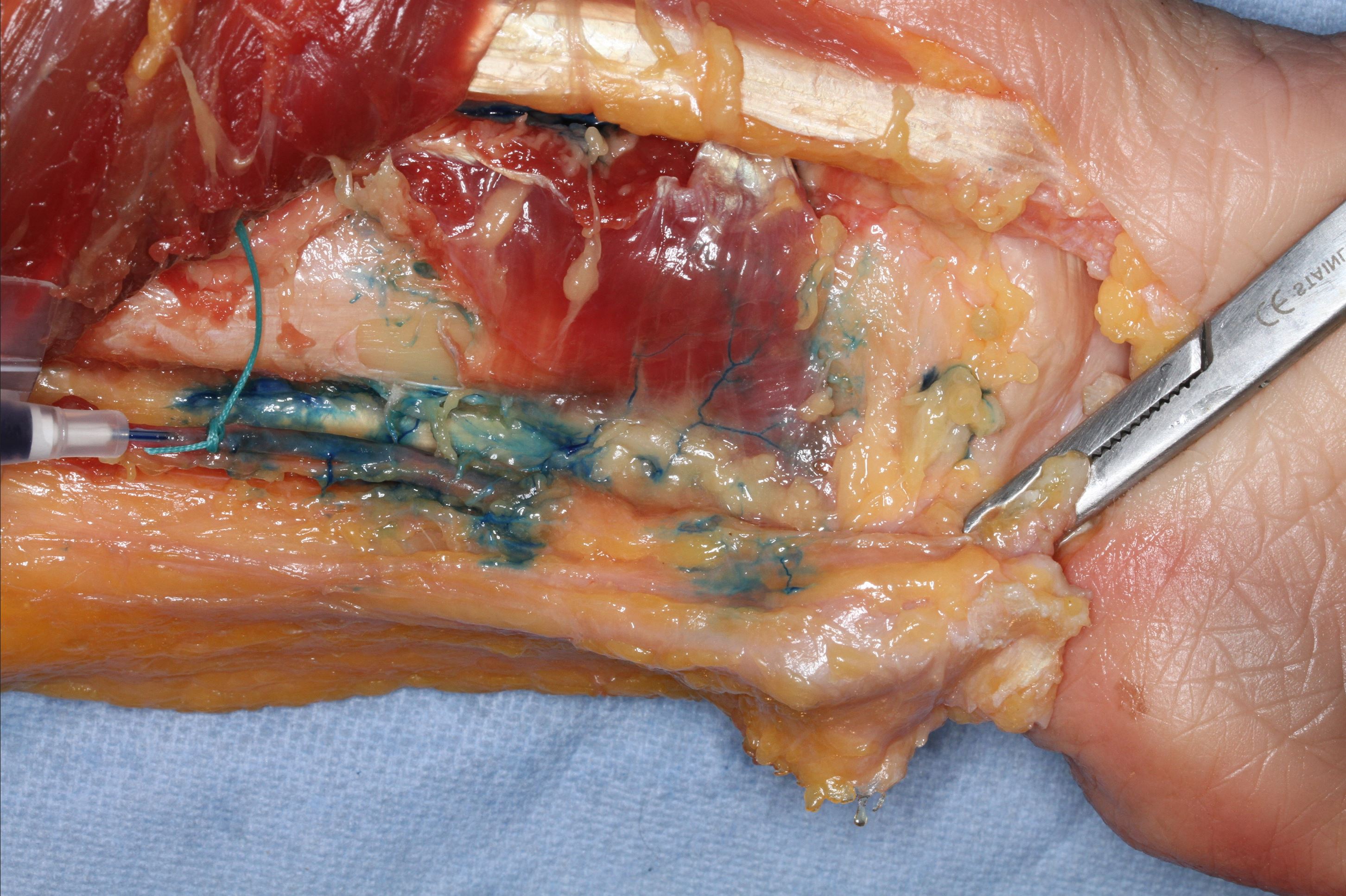
Figure 1. The pronator quadratus muscle perforators of the radial artery in the right forearm are stained with methylene blue.
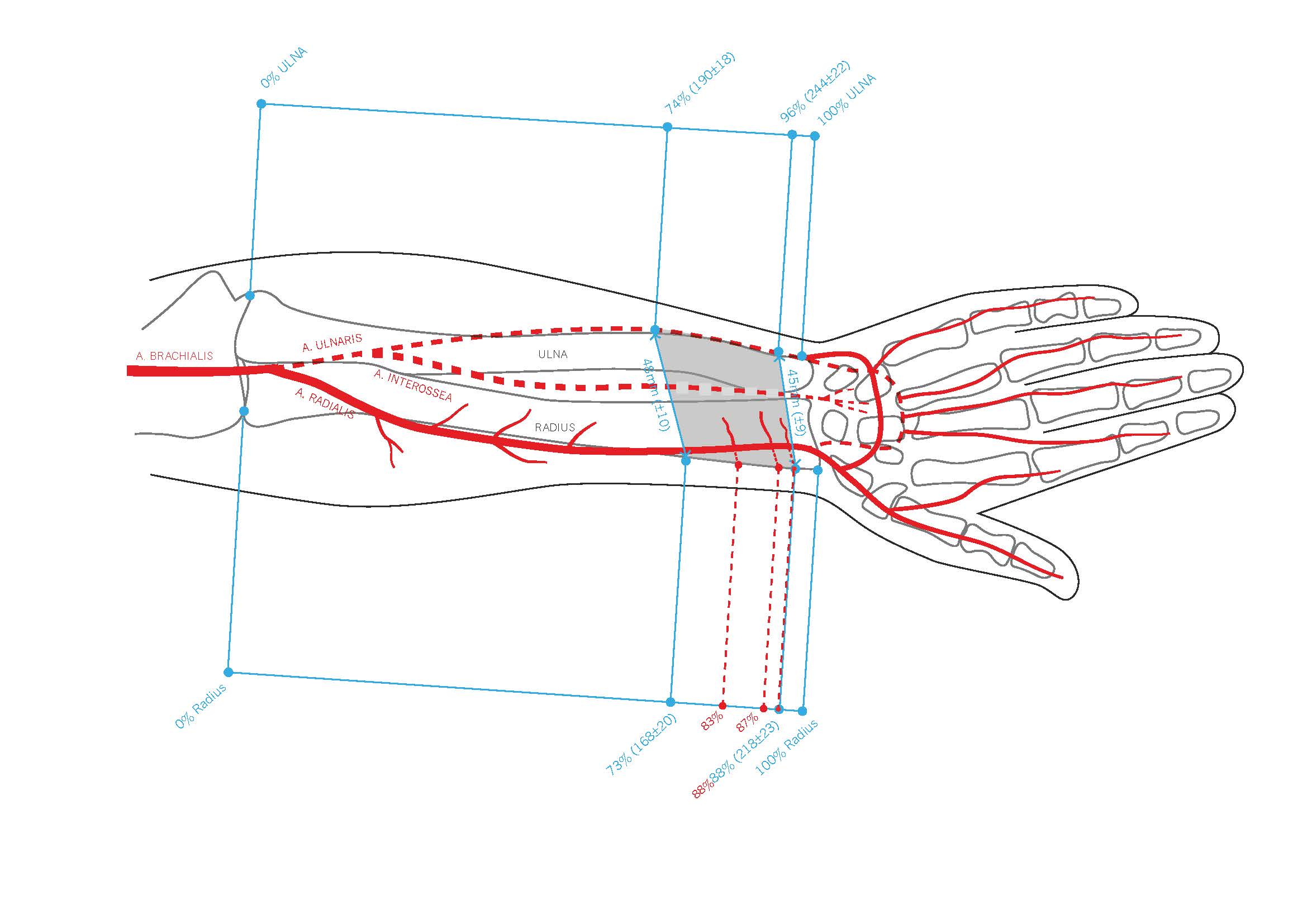
Figure 2. This comprehensive illustration provides a detailed view of the pronator quadratus muscle (PQM), its blood supply, and the relative positions of the radius and ulna within the right forearm. The PQM is highlighted, with the entire lengths of the forearm bones (radius and ulna) marked in grey and outlined in blue. The proximal border of the PQM on the radius is marked at 73%, while the distal border is marked at 88%. Similarly, on the ulna, the proximal border of the PQM is marked at 74%, and the distal border at 90%. Additionally, the origins of the perforators from the radial artery are indicated in red at 83%, 87%, and 88%.
Furthermore, a correlation was observed between the length of the radius and the location of the first radial muscle branch, as indicated by the Pearson Correlation coefficient: the first muscle branch branched out from the radial artery with an increase in the length of the radius bone. However, no correlation was found between the weight or length of the PQM and the number of its muscle branches.
We confirm the innervation of the PQM by the anterior interosseous nerve (AIN). Measured from the radial head, the AIN originated between the first and second proximal quarter of the radius bone, with an average proximal measurement from the radial head at 52 ± 12 mm (23 ± 4%) and an ulnar measurement from the olecranon at 79 ± 17 mm (31 ± 5%) (Table 4).

The therapy goal of facial reanimation surgery is the reconstruction of static and emotional dynamic symmetry [13–16]. The diversity of facial reanimation procedures indicates the individualized treatment concepts that account for the specific patients’ symptoms and deficits [17]. Iatrogenic causes are the most common etiologies of facial palsy [18]. Facial nerve injuries may manifest after the surgical removal of cerebellopontine tumors, acoustic neuromas, parotid masses, or the development of tumors along the path of the facial nerve [18]. In certain instances, iatrogenic facial palsy arises from deliberate R0 tumor resections, characterized by the achievement of clear margins at both macroscopic and microscopic levels. Conversely, there are situations where postoperative facial nerve deficits occur unexpectedly [18]. In oncological cases, postoperative facial palsy needs to be differentiated from preexisting facial weakness caused by the tumor. Neoplastic causes account for around 5% of facial palsy cases and are usually characterized by gradual onset of facial weakness [19,20]. In cases of oncological facial nerve resection, the timing of facial nerve repair and facial reanimation are crucial, as denervated facial muscles undergo atrophic changes which limit their potential for reinnervation [21,22]. Direct nerve repair, early interposition nerve grafting [23], even in proximal injuries close to the brainstem during cerebellopontine tumor surgery [21,24,25] and other facial reanimation procedures provide the optimal basis to regain facial nerve function. Additionally static procedures are recommended to be used in combination with dynamic facial reanimation to improve facial function in patients with significant comorbidities and limited life expectancy who are not suitable for complex multi-stage reconstructions [26]. Even in cases with planned postoperative radiation, complication rates following static procedures are low [27]. Moreover, in oncological resections, treatment planning not only has to address reinnervation and the restoration of missing mimetic muscles, but also consider soft tissue reconstruction for possible radiation therapy [16,28].
Reports of immediate facial reanimation techniques that combine the reconstruction of soft-tissue skin defects with simultaneous functional neuromuscular reconstruction during oncological tumor resections are quite rare. The lateral circumflex femoral system has been utilized for repairing extensive defects in the head and neck regions, aiming to restore both facial aesthetics and dynamic function [29,30]. The surgical anatomy of the anterolateral thigh flap has been meticulously studied in the past [30–34]. Recent publications have described the innovative use of a chimeric approach, involving the transplantation of the PQM in combination with a radial forearm flap [12,35]. This technique has shown promising results for reanimating paralyzed faces without causing donor site morbidity.
Anatomic studies of the PQM showed a two-headed muscle. The superficial head of the muscle is more distal; it originates from the distal fourth of the ulna and inserts with its transverse fasciculi into the distal fourth of the anterior surface of the radius bone. The deep and more proximal head of the PQM has oblique fasciculi extending from the proximal ulnar origin to the distal radial insertion. The origins of both heads are slightly apart, approximately 2 mm apart [36]. The superficial head is the prime mover in forearm pronation, whereas the deep head functions as a dynamic stabilizer of the distal radioulnar joint [37].
The muscle is innervated by the AIN, which arises from the median nerve at the radiohumeral joint line. AIN runs on the anterior surface of the interosseous membrane of the forearm and passes posterior to PQM, dividing into three or four terminal branches [38]. The nerve is accompanied by the anterior interosseous artery, which is the supplying artery of the PQM.
Due to the inadequate description of anatomic landmarks for the PQM in the literature, this study was conducted to elucidate the anatomic features of PQM and its vascularization by the radial artery for clinical applications. The findings revealed that the average weight of PQM was 8 ± 3 g. This muscle, shaped like a trapezoid, had an average length of 50mm and a mean width of 45mm distally and 48mm proximally. On the radius, the proximal border of PQM averaged at 169 ± 21 mm from the radial head, which corresponds to 73 ± 3% of the radial bone length, and the distal border was at 218 ± 24 mm (95 ± 3%). On the ulnar aspect, the proximal and distal borders of PQM were 190 ± 18 mm (74 ± 4%) from the olecranon and 244 ± 23 mm (96 ± 3%), respectively (Table 1 and Table 2). In addition to the detailed measurements of the muscle's insertion and origin, as well as the origin of the anterior interosseous nerve, we established that PQM receives its blood supply from both the anterior interosseous artery and the radial artery. Up to four muscle branches originating from the radial artery supply PQM. There was no correlation found between the weight and size of PQM and the number of radial muscle branches. PQM is innervated by the AIN and can be harvested at length for nerve reconstruction. The origin of this nerve can be located between the first and second proximal quarters of the radial or ulnar bone.
Although a former study revealed differences between male and female specimens in connection with significantly greater radial-ulnar width (p = 0.005), area (length times width; p = 0.006), and volume (p = 0.033) of the PQM in male specimens, as well as a greater distance from the radial styloid to the distal arborization of the AIN (p = 0.005) compared with female specimens [39], we could not find any significant distinction in correlation to weight (p = 0.46) or length (p = 0.2) of male and female muscles.
The PQM is known to be active during grip strength. The superficial head of PQM contracts more actively when the forearm is in pronation, whereas the deep head constantly contracts in all positions. Both heads play a role in grip strength, with the superficial head contributing to pronation strength [40]. The attachment of PQM to the base of the ulnar styloid process is considered an important structure that prevents the head of the ulna from impacting against the carpal bones [38]. Despite these crucial anatomic functions of PQM, there have been no reports of donor site morbidity after harvesting PQM in the past [12,35,41].
One of the limitations of our study is the restricted number of specimens, which prevented us from correlating sex and laterality (dominant right or left hand) with the size/weight of PQM. A general limitation of anatomical studies is the inability to assess muscle function.
In this study, our aim was to analyze clinically relevant anatomical landmarks of the PQM and its blood supply. Our findings can provide surgeons with valuable information for specific planning and treatment, especially when utilizing PQM for reanimating a paralyzed face.
The combination of the pronator quadratus muscle with a radial forearm flap presents a safe and feasible alternative for facial reanimation, especially for reanimating a paralyzed face. There are up to four radial artery muscle perforators, each under 0.5 mm in diameter, that arise from the radial artery at the origin of the PQM. These perforators ensure a consistent blood supply to the PQM when elevated with a free radial forearm flap, forming a chimeric flap.
Received date: July 17, 2023
Accepted date: September 25, 2023
Published date: November 23, 2023
We would like to express our gratitude to Maria Prieto Barea for creating the figure (https://www.mariaprietobarea.com). We have obtained the necessary permission for the use of the figure in this publication.
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2023 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.

Most isolated syndromes of Eight-and-a-Half Syndrome are associated with vascular etiology. Symptomatic trigeminal neuralgia due to infarction is rare. The author reports a patient with left-sided facial pain. It was followed by one-and-a-half syndrome with facial nerve palsy during the next day. Diffusion-weighted magnetic resonance imaging of his head revealed restricted diffusion in the left inferior pontine tegmentum neighboring the fourth ventricle extending ventrally. This case is the first report of Eight-and-a-Half Syndrome presented with recurrent attacks of unilateral facial pain, fulfilling criteria for classical trigeminal neuralgia.
This case report describes authors successfully attempt to perform a topographically correct sural nerve transplantation in the extracranial facial nerve stem using the intraneural facial nerve stem topography proposed by Meissl in 1979. This is the first reported case of successful fascicular nerve grafting of the facial nerve stem following extensive laceration.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
Facial palsy affects both the functional and aesthetic aspects of the face. Many techniques for both static and dynamic facial reanimation have been described. Authors present their series of facial reanimation using the functioning free gracilis muscle flap innervated by the masseteric nerve.
The pronator quadratus muscle flap offers the plastic surgeon an intriguing additional option for reconstruction. Your work further highlights the anatomical basis of this flap, previously described both as a pedicled and a free flap. This holds true for applications spanning from carpal reconstruction to upper extremity interventions, as well as the complex realm of facial reanimation and addressing facial defects. While the bulk of the literature predominantly delves into the primary pedicle stemming from the anterior interosseous artery, there are instances wherein the flap's foundation on the radial artery is also detailed. Nevertheless, your article substantially augments the understanding of the anatomical fundamentals governing the flap's viability. Consequently, its potential for publication is evident, underscoring the need for a meticulous review of the references section. Furthermore, a considered acknowledgement and emphasis on the prior contributions of fellow scholars in the discourse is recommended. In essence, this research epitomizes an avant-garde in anatomical and vascular scholarship, bringing to the fore the symbiotic relationship between the pronator quadratus muscle and the radial forearm flap in the realm of facial reanimation. As we grapple with the challenges posed by facial paralysis, the ramifications of this pioneering study resoundingly advocate for its rightful place within esteemed medical literature after some minor issues addressed.
In a pioneering investigation into the intricate anatomical marvels within the human form, this study meticulously deciphers the nuanced interplay between the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). This convergence carries profound implications for the restoration of facial animation, which becomes compromised by paralysis—a condition that casts shadows over both emotional resonance and functional dynamism. The exhaustive analysis elucidates the complex fabric of the PQM, highlighting its vascular pathways emanating from the tributaries of the radial artery—a cornerstone revelation in the realm of facial reanimation methodologies. Employing precise dissection methodologies, the authors embarked on a comprehensive journey through hemi-facial cadaveric segments, unveiling the PQM's dimensional intricacies, its alliance with pivotal osseous landmarks, and the surrounding vascular terrain. This rigorous study delineated the PQM's unique trapezoidal geometry, characterized by diverse measurements. Moreover, the revelation of numerous muscular branches stemming from the radial artery as vital conduits, coupled with the identification of the anterior interosseous nerve (AIN) as its neural emissary, augments our comprehension of the anatomical landscape. Considering these perspectives, I advocate for its publication.
While this narrative is painstakingly crafted, with a balance between precision and limpidity, it does not shy away from acknowledging subtleties. Its assertion, implying a pioneering stance in untangling the complexities of flap anatomy, beckons meticulous scrutiny. Historically, the annals of anatomical and surgical scholarship have traced the trajectory of flap delineation, from its inception in upper limb reconstruction to its refined role in facial rejuvenation techniques. The pivotal role of the anterior interosseous artery in the flap's genesis has been a focal point, and a smattering of antecedent studies have delved into the dual facets of the radial artery, harmonizing theory with practice. However, the contemporary insights presented in this manuscript carve a unique niche within academic dialogues. To elevate its stature further, a rigorous augmentation of references is imperative. This endeavor should interweave with an acknowledgment of past luminaries, bestowing tribute upon their foundational contributions and fostering a coherent scholarly discourse. Incorporating such pivotal keywords and terminologies enhances its digital footprint, beckoning discerning academic minds with its intricately woven narrative. It orchestrates a symphony of erudition, destined to reverberate amidst the evolving paradigms of science and technology.
RevisionThe authors appreciate the reviewer's thorough assessment of their manuscript and their recognition of the meticulous exploration of the pronator quadratus muscle and the radial forearm flap's interplay. The authors value their advocacy for the publication and understand the need to enhance the references, acknowledging past contributions to the field. The authors aim to integrate these valuable suggestions into their work to further enrich its scholarly discourse and digital presence. The reviewer's insights will undoubtedly strengthen the manuscript's position within the evolving landscape of anatomical research.
In a pioneering exploration, this study intricately unravels the anatomical and vascular complexities inherent in the utilization of the pronator quadratus muscle (PQM) in conjunction with the radial forearm flap (RFF) to restore facial animation in cases of paralysis. The ramifications of facial paralysis are profound, notably impacting emotional expression and functional efficacy. Through comprehensive analysis, this investigation sheds light on the intricate structure of the PQM, emphasizing its vascular supply derived from the radial artery's tributaries—an invaluable insight for advancing facial reanimation techniques. Employing meticulous dissection techniques, researchers methodically examined hemi-facial segments from cadaveric samples, unveiling both the dimensional specifics of the PQM and its relationships with crucial bony landmarks and the encompassing vascular landscape. This painstaking analysis reveals the trapezoidal configuration of the PQM, characterized by diverse metrics. The discovery of multiple muscular branches of the radial artery as vascular suppliers, along with the confirmation of the anterior interosseous nerve (AIN) as its neural conduit, further enhances our understanding. Ultimately, this research signifies a cutting-edge contribution to anatomical and vascular scholarship, highlighting the symbiotic interaction between the pronator quadratus muscle and the radial forearm flap in the realm of facial reanimation. This article merits acceptance, given its substantial and valuable insights that stand to enrich the broader medical discourse.
RevisionThe authors are genuinely grateful for the reviewer's insightful assessment of the study, recognizing its pioneering nature in exploring the utilization of the pronator quadratus muscle (PQM) with the radial forearm flap (RFF) for facial animation restoration in paralysis cases. The appreciation of the study's impact on emotional expression and functional efficacy in facial reanimation is truly valued. The reviewer accurately captures the essence of the comprehensive analysis, emphasizing the PQM's intricate anatomical and vascular complexities, including its trapezoidal configuration and multiple muscular branches from the radial artery. The authors are deeply honored by the reviewer's endorsement of the research's cutting-edge contribution to anatomical and vascular scholarship, and the authors remain committed to addressing any further suggestions to ensure the manuscript's excellence in enriching the medical discourse.
Tzou CHJ, Steinbacher J, Meng S, Metz A, Oliva S, Weninger WJ, Rodriguez-Lorenzo A. Chimeric radial forearm flap with pronator quadratus muscle for facial reanimation: An anatomical feasibility study. Int Microsurg J 2023;7(1):3. https://doi.org/10.24983/scitemed.imj.2023.00176