Emerging anatomical concepts challenge microsurgical dogma. The current anatomy of the skin and subcutaneous tissue was reviewed with the objective of challenging the existing understanding of fasciocutaneous microanatomy using an updated anatomical model. Numerical anatomical data were compiled and utilized to create an updated and scaled model, defining integumentary neuroarterial, venolymphatic, and connective tissue systems. Additionally, a second model detailing the neurovasculature of the head and neck is presented, illustrating the relations of perforator arteries. Microangiosomes, the strength of their connections, and their relation to dissection planes are described. Clinically relevant structures are outlined, along with general principles and regional variations. We explore the viability of dermal plexus flaps and their potential for engraftment through plexus-to-plexus apposition. A comparison is drawn between subdermal and deep-dermal plexi. Furthermore, the peculiarities of head and neck perfusion and lymphatic drainage are discussed. These models inform our approach to dissection planes, fluid injection depths, flap viability, neurotization, post-inflammatory hyperpigmentation, tissue engraftment, debulking, and head and neck lymphatic drainage. This illustrated review offers an updated understanding of fasciocutaneous microanatomy and how to safely utilize it.
In the microsurgical era, the distinction between flaps and grafts has become blurred. The success of thin pure-skin flaps [1] and thick skin-fat composite grafts [2] reflects a growing command over microvascular anatomy. Nevertheless, some erroneous concepts persist that can jeopardize tissue viability and patient safety. Dermal plexus flaps are used to re-drape entire limbs [3–5], show a limited area of perfusion on perforator imaging [1], and often suffer marginal necrosis during excisional debulking [6,7]. Certain grafts become vascularized within 24 hours through direct anastomoses between remnant vessels [8]. These paradoxes expose the need to update our understanding of cutaneous microvasculature to better inform surgical practice.
This study aims to redefine current understanding of fasciocutaneous microanatomy by challenging prevalent concepts with an updated anatomical model. Specifically, it explores microangiosomes, compares the dermal plexi, and investigates head and neck perfusion and lymphatic drainage. The objective of this research is to enhance microsurgical techniques and improve patient outcomes by providing insight into the limitations of existing procedures across various clinical scenarios.
To develop the primary model, we purposively retrieved articles on anatomy of each component of the integumentary system from PubMed (MEDLINE), Scopus and Google Scholar from January 1970 to April 2023 (initial search conducted through December 2022). The keywords used include ‘skin’, ‘cutaneous’, ‘integument’, ‘subcutaneous’, ‘microanatomy’, ‘microscopy’, ‘vasculature’, ‘artery’, ‘vein’, ‘lymphatic’, ‘perforator’, ‘perfusion’, ‘neuroanatomy’, ‘nerve’, ‘innervation’, ‘melanocyte’, ‘connective tissue’, ‘collagen’, ‘fascia’, ‘adipose’, and ‘fat’. Secondary retrieval was done using a citation-networking software (ResearchRabbit, Version 2.0, Human Intelligence Technologies, Incorporated) until we achieved concept saturation. We only included studies on human skin and/or subcutaneous anatomy, which observed at least 5 tissue samples. We excluded studies which were simulation-based, discussed only post-surgical imaging, reported unoriginal concepts, or those deemed not surgically relevant, as determined by consensus between two reviewers. In cases of ambiguity, the senior reviewer’s decision was solicited. Objective data and images were used to prepare a scale model. In our reporting, we emphasized the structure of microangiosomes, the strength of plexus connections, and their relation to dissection planes. In the discussion, we explore the role of the updated model in clarifying our understanding of aspects of microsurgery, specifically dissection planes, injection depths, flap viability, neurotization, post-inflammatory hyperpigmentation, tissue engraftment, debulking, and head and neck lymphatic drainage.
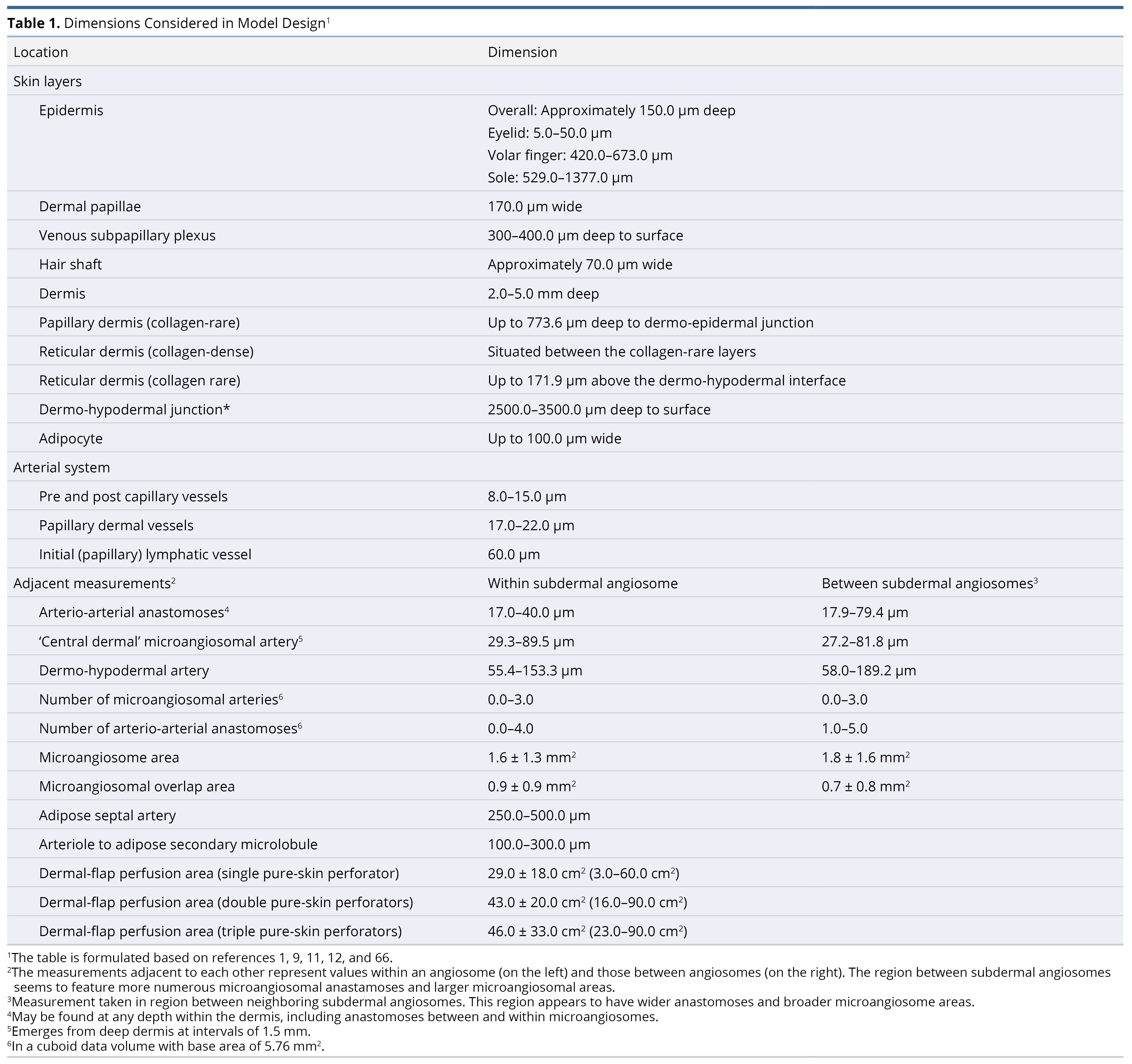
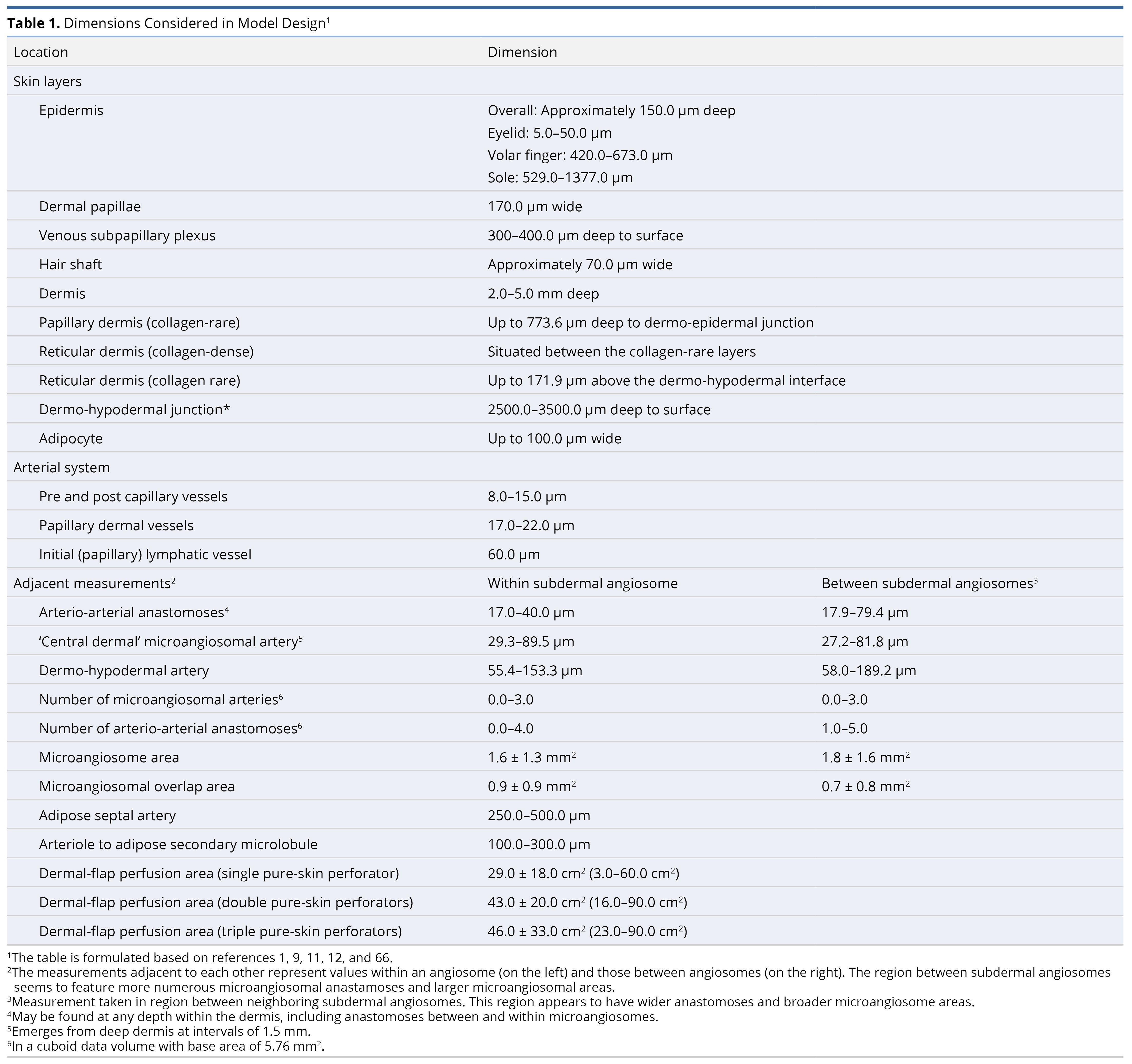
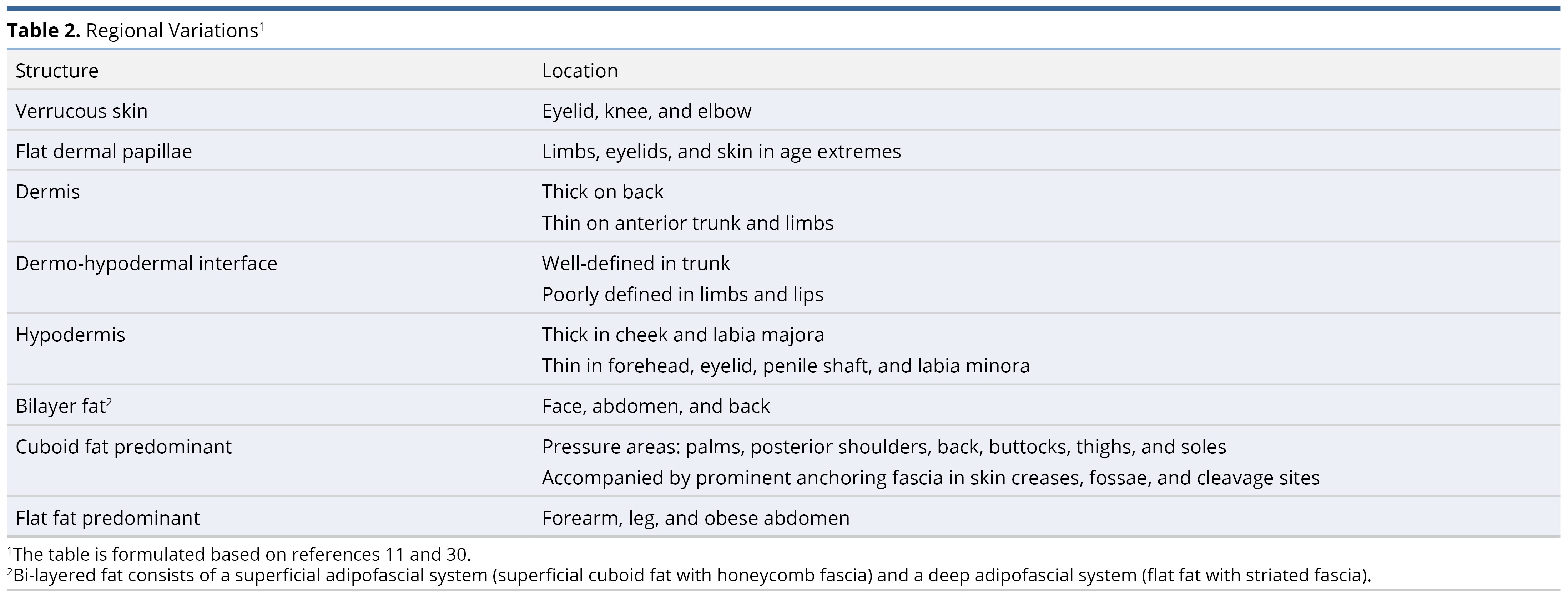
To prepare the primary model (Figure 1), our literature review comprised 22 original articles and reviews, focusing on the anatomy of connective tissue [9,10], the adipofascial system [11–14], lymphatics [10,15], veins [10], special organs [16], and arteries along with their accompanying nerves [1,10,17–29]. Among these, 9 were most essential in preparing the final model [9–11,14,15,18,21,24,27,29]. Measurements of structures are compiled in Table 1. For clarity, the dermal papillae are depicted as wider, while the deeper plexi have not been fully elaborated. Regional variations are summarized in Table 2 [11,30]. Individual systems are detailed below, and their surgical implications are reviewed in the discussion section.

Figure 1. A scale representation of the human integumentary system (30:1, with a hair shaft width as a 70 µm reference). Connective tissue (1-17, colored in cyan for collagen fibers and beige for adipose): 1, epidermis; 2, dermis; 3, protective adipofascial system; 4, lubricant adipofascial system; 5, rete peg; 6, dermal papilla; 7, papillary dermis (collagen rare); 8, integumentary ligament; 9, reticular/deep-dermis (collagen-dense layer); 10, adipose tissue in dermis; 11, reticular/deep-dermis (collagen-rare layer); 12, cuboid fat; 13, honeycomb fascia; 14, superficial membranous fascia; 15, striated fascia; 16, flat fat; 17, deep membranous fascia. Lymphatics (18-22, colored in green): 18, initial lymphatic (open-ended, avalvular channel); 19, pre-collector lymphatic (has valves); 20, lymphagion (channel between valves) of a collector lymphatic (has circumferential contractile cells); 21, semilunar valve; 22, pre-nodal lymphatic trunk. Veins (23-34, colored in deep blue): 23, venule; 24, subpapillary venous plexus; 25, semilunar valve (start in deep dermal layer); 26, deep dermal plexus; 27, subdermal plexus; 28, peripheral tributary; 29, interlobular septal vein; 30, suprafascial plexus; 31, superficial fascial perforator; 32, subfascial plexus; 33; venous tributaries (peripheral/septal); 34, deep fascial perforators. Special organs (35-47): 35, Meissner corpuscle; 36, Merkel disc; 37, free nerve endings; 38, sebaceous gland; 39, arrector pili muscle; 40, Ruffini ending; 41, lanceolate-ending receptors; 42, Pacinian corpuscle; 43, Krause end bulb; 44, glomus body (closely related to 42); 45, hair bulb and hair-end nerve plexus; 46, eccrine sweat gland; 47, sudomotor plexus. Arteries (48-60, colored in red): 48, terminal dermal arteries giving off papillary capillaries; 49, epineural arterial complex; 50, inter-microangiosomal anastomosis (rare); 51, intra-microangiosomal anastomosis (rare); 52, ‘central dermal’ microangiosomal artery; 53, deep dermal plexus; 54, subdermal plexus; 55, descending adipofascial artery; 56, anastomoses of the deep fatty layer; 57, deep fascial perforators; 58, septal artery; 59, nervi arteriosum; 60, vasa nervosum. Nerves (61-64, colored in yellow): 61, subpapillary neve plexus; 62, dermal nerve trunk (whose branches follow dermal arteries); 63, deep dermal plexus; 64, cutaneous nerve and anastomoses. This figure is an original creation by the first author, prepared for this publication.
Melanocytic System
The basal layer of the epidermis houses melanocytes. These neural crest cells have a dermatomal distribution [31]. They are also found in hair bulbs, and perhaps in sebaceous glands [32]. Melanocyte density doubles in the rete ridges as compared to the inter-ridge area [33,34]. Thus, thicker grafts have exponentially more melanocytes.
Connective Tissue System
The mechanical properties of skin and its dissection planes are of surgical relevance. The epidermis (Figure 1 label #1) is of variable thickness (Table 2) [30]. The undulating dermo-epidermal interface contributes the most to skin shear-resistance [10]. This interface is flat in extremes of age, contributing to easy bruising. Additionally, aged skin exhibits dermal thinning from keratinocyte apoptosis and senescence, fewer blood vessels, and larger, albeit hypofunctional, sebaceous glands [35]. Photoaging independently reduces shear-resistance and contributes to dermal thickness changes. Photoaging affects sun-protected skin (e.g., torso) more as compared to sun-exposed skin (e.g., forearm or calf) [36]. Integumentary ligaments anchor rete ridges to deeper layers (Figure 1 label #8). These ‘retaining ligaments’ are prominent in the unaged face, and in patients with early Dupuytren’s hand contracture [11,37,38]. They transmit contractions of superficial muscles to the skin, while protecting the vessels.
There are three distinct densities of the dermal extracellular matrix [9]. The superficial (papillary) dermis (Figure 1 label #7) is collagen-and-elastin-rare. The middle (upper reticular) dermis is the thickest layer and is uniformly dense (Figure 1 label #9). The deepest 0.18 mm of the reticular dermis (Figure 1 label #11) is also rare. The dense middle dermis makes intradermal injection difficult. It reflects injected fluid back up, raising the papillary dermal plane [39]. This layer may act as a barrier to the transmission of infiltrated fluids (anesthetics, fillers, etc.) across planes, whether injected intradermally or subdermally [40].
Regional variations in connective tissue lead to anisotropy in skin tension lines [41,42]. Collagen-elastin interplay accounts for skin biomechanical properties, like non-linear deformation, anisotropy, and viscoelasticity [43]. Collagen provides an excellent surface for early fibrin-mediated adherence of wound edges, and for late biointegration [44]. Thicker grafts are less likely to contract [45,46], and their scar sheet may enhance tissue strength [47].
Adipose Tissue
Subcutaneous white adipose tissue comprises the superficial/protective and deep/lubricant adipofascial systems [11]. The superficial system consists of densely packed adipocytes that are tethered between the superficial fascia and dermal integumentary ligaments by the honeycomb fascia (Figure 1 label #12, 13). It has a cushioning effect. The deep system has loose striated fascia, which allows planes to glide smoothly (Figure 1 label #15, 16). These systems are phenotypically distinct. The superficial system serves a fat metabolism function, while the deep system is pro-inflammatory [14]. The adipofascial system lends pliability to skin-fat composite grafts [2]. Their distribution differs across the body (Table 2).
Dense aggregates of adipocytes form secondary microlobules. These receive end-arterial supply (Figure 1 label #12). Superficial fat arteries are smaller, albeit more numerous, than those in deep fat [14]. Deep fat receives additional perfusion from descending branches of deep dermal and subdermal plexi (Figure 1 label #56) [14,18]. Our clinical observation is that fat necrosis predominantly involves deep fat. While it is the better-perfused layer, cells in the deep fat overexpress cell-death genes [14].
Microlobules receive central end-arteries and are peripherally drained along septae (Figure 1 label #33). Arterial pathology primarily affects the lobule (lobular panniculitis), and venous disease affects the septal and paraseptal areas (septal panniculitis) [12]. In degloving trauma, the shearing action severs perforators ascending to the deep fat (Figure 1 label #57) [48].
In some regions, like the thigh, there are multiple adipofascial layers [13]. In obesity, adipose expansion and fibrosis lead to the formation of more adipofascial layers, separated by pseudo-superficial fascia (thickened honeycomb fascia) (Figure 1 label #13) [49]. Deep fat predominates in abdominal obesity (Table 2).
Venolymphatic System
Till recently, it was assumed that there exist subpapillary venous, arterial, lymphatic, and nervous plexi [10,23,27]. High-resolution episcopic microscopy studies confirm the presence of a subpapillary venous plexus and the absence of an arterial one (Figure 1 label #24, 61) [21,24,25]. Relevant venular physiologic phenomena include the venoarterial reflex (venous distension prompts systemic vasoconstriction), the venuloarteriolar reflex (venular distension prompts regional arteriolar constriction), and the Bayliss effect (venular distention prompts mural venous constriction) [50].
Pre-nodal lymphatics consist of 4 distinct types of channels (Figure 1 labels #18–22) [15]. They traverse collagen-rare planes, into which large proteins readily drain (Figure 1 label #19). Tissue edema pulls tethers that maintain lymphatic channel patency and open drainage pores. Like veins, their semilunar valves emerge in the deeper dermis (Figure 1 label #21). Lymphatics do not directly drain fat (Figure 1 label #22), hence the necessity of burn escharotomy. Veins and lymphatics are closely related [10]. Cellular plasticity, lymph node shunts, and dissection studies suggest the natural occurrence of macrovascular lymphatic/blood linkages [15,51].
In the head and neck, lymphatic drainage is complex. Collecting ducts from a single site drain to multiple different nodal basins [52,53]. These ducts travel laterally, towards the scalp and lateral face and neck [53,54]. Injury to these ducts results in prolonged edema, which requires about 3 weeks for repair [55]. The superficial and deep lymphatic system sandwich the superficial musculoaponeurotic system (SMAS) [54]. Valveless interconnections run between the two. Surgical insult to either system can result in prolonged edema [54].
Neuroarterial System
Cutaneous nerves and arteries are closely related [10,22]. Dermal sensory nerve bundles arborize like arteries (Figure 1 label #62) [25,29]. Superficial fascial and subdermal neurovascular ‘freeways’ parallel specific cutaneous nerves [56]. These vascular axes begin with arteries (e.g., descending genicular artery), distally make true anastomoses with long axial perforators (e.g., from the posterior tibial artery), and run in parallel to specific large nerves (e.g., saphenous nerve) [22,56]. These nerves are slightly apart from the arteries and can be separated. However, their inclusion in flaps increases the chances of preserving the vascular axis. Along cutaneous nerves, even choke vessels are relatively large [56].
The papillary dermis has a rich capillary supply, though no true plexus exists here [10,23]. Papillary perfusion is thermoregulated. In the reticular dermis, flow is metabolism/hypoxia-mediated [40,41].
Contrary to previous descriptions [1,18], the deep dermal plexus is distinct from the subdermal plexus (Figure 1 labels #53 and 54) [9,21,24]. It is random patterned, unlike the more axial subdermal plexus [18,27]. Its ascending vessels perfuse small 'microangiosomes' (see Table 1 for areas) [1]. Microangiosomes do not form any plexus, only a few insignificant anastomoses (Figure 1 labels #50–52) [21,24]. At the border of adjacent subdermal angiosomes, neighboring microangiosomes have slightly larger anastomoses and territories (see Table 1) [24].
Subdermal angiosomes are demarcated by ‘choke vessels.’ These are small-caliber regulatory vessels. They dilate (arteriogenesis) under the influence of vasodilators, high flow, and vascular delay [22,43]. Angiosomes hardly cross scar lines [17,20,57].
Deep fascial perforators are consistently found within anchoring/fixed connective tissue planes, like at the modiolus. Here, perforators are protected from shear stress and have a shorter course to the skin. Perforators may be cutaneous, septocutaneous, or musculocutaneous. They respectively supply axial, fasciocutaneous, and random-pattern flaps, though there are many exceptions to this nomenclature [43]. Perforator diameter relates to tissue mobility and laxity [22]. In the face, arterial perforators are larger and closer to veins caudally compared to cranially [58].
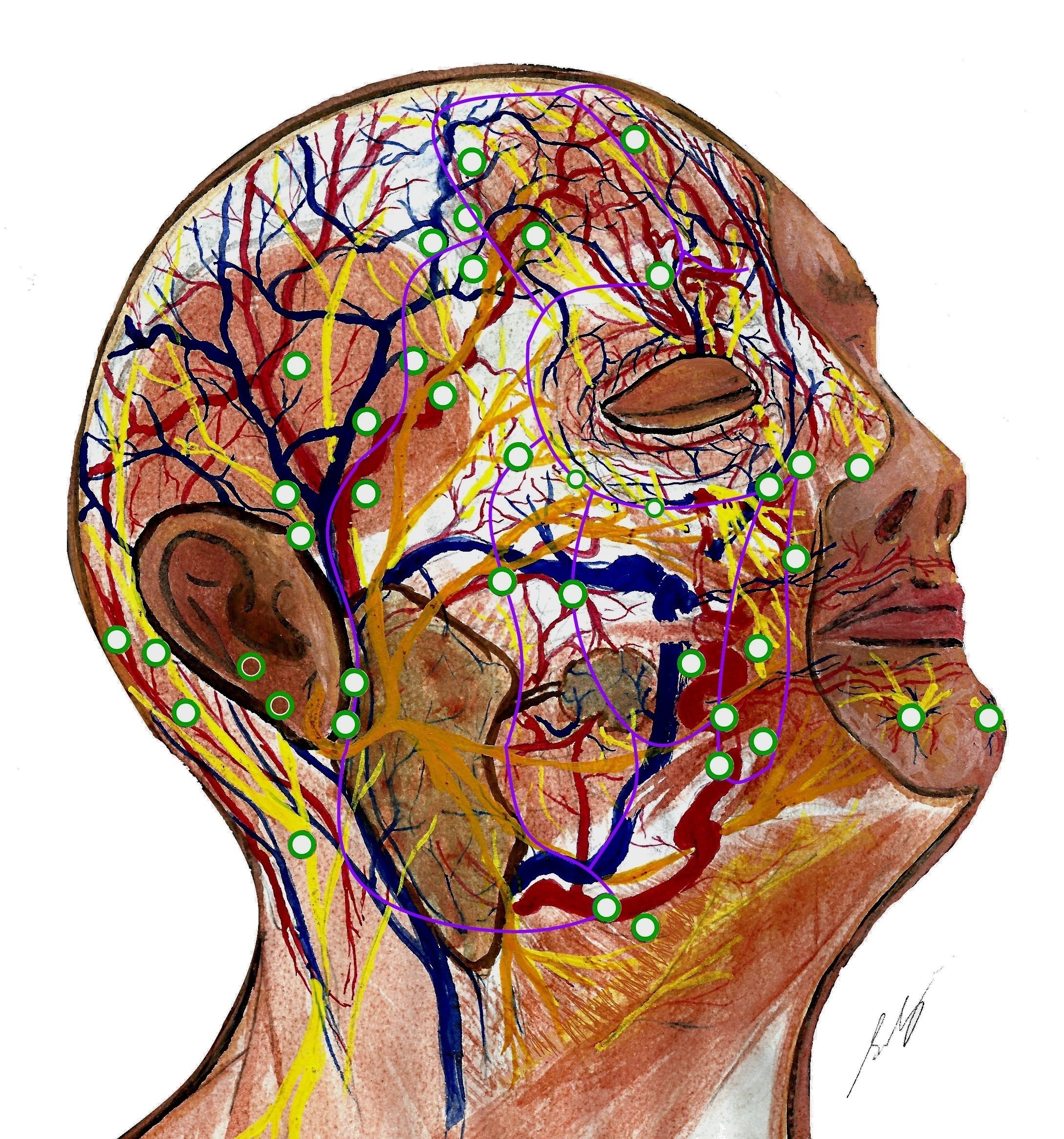
Head and neck neuroanatomy is complex. Perforators mostly arise from the facial, superficial temporal, and supratrochlear arteries, along fascial planes [38,59]. There are true midline anastomoses in the lips [59], but choke anastomoses across the forehead [60]. Dense arterial plexi exist deep to, within, and superficial to the SMAS. The subdermal plexus is particularly rich in the ‘blush regions’ (malar area and anterior neck) [60]. Veins travel distant from arteries in the nasolabial area, forehead, and scalp [61]. Recent studies suggest glabellar flaps can include longer vessels (paracentral artery and central artery) and larger veins (central vein) than paramedian flaps (based on the supratrochlear artery) [62–64]. However, these central arteries may be absent in some patients. There exist communications between midfacial sensory (infraorbital) and motor (facial) nerve trunks, located around 16 mm lateral and 6 mm superior to the alar rim (Figure 2) [65]. This region may provide an alternate pathway for sensory and motor neurotization, i.e., a ‘babysitter nerve’, and should be safeguarded. Relations between facial perforators and surrounding neurovasculature are understudied compared to limbs. Figure 2 depicts the current anatomy of facial perforators and their relation to sub-SMAS neurovasculature.
Special Organs
The special organs in the skin (Figure 1 labels #35–47) are well-reviewed by Metze et al. [16]. Glomus bodies (Figure 1 label #44) shunt deep dermal arteries into veins. They are present in distal extremities alongside Pacinian corpuscles (Figure 1 label #42). This suggests that glomal perfusion is neuroregulated [16].
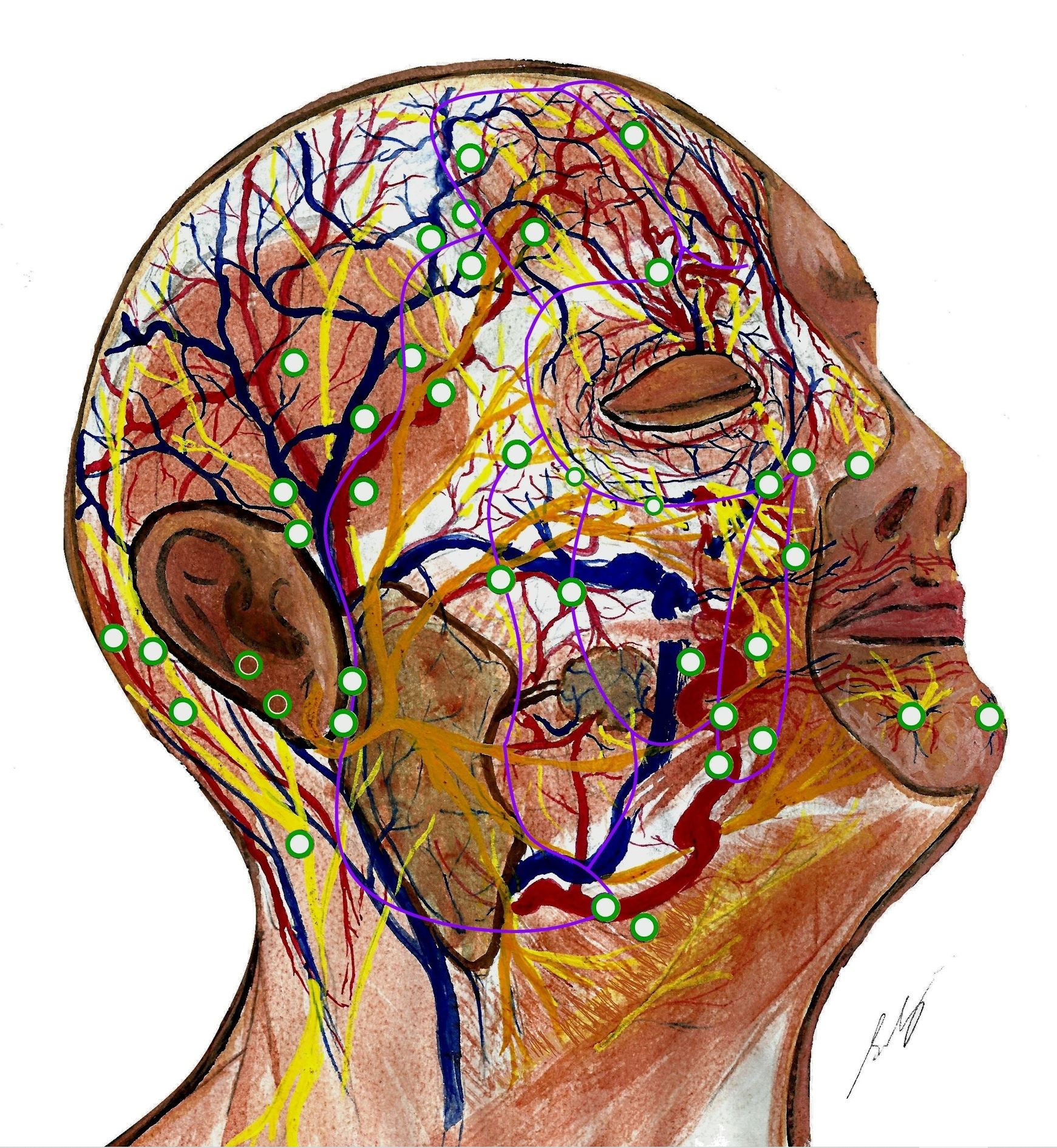
Figure 2. Facial neurovasculature with perforators mapped. Perforators (green) originate where dominant vessels traverse fixed connective tissue planes (purple). Perforators supply the superficial musculoaponeurotic system (SMAS) and the supra-SMAS fat compartments and skin. They are longer in the more mobile lateral face, and shorter and more clustered medially. Perforators often enter SMAS alongside nerves. Veins generally travel apart from arteries in scalp, forehead, and nasolabial regions. The genu (knee) of the supratrochlear artery is shown, as it emerges from corrugator supercilii. The depiction shows the communication between the zygomatic branch of the facial nerve and the infraorbital nerve, which is located superolateral to the alar rim. Dominant arteries (left to right) include occipital artery, posterior auricular artery, superficial temporal artery, frontal branch of superficial temporal artery, supratrochlear artery, facial artery, and mental artery. Some perforators also arise from the transverse facial artery, zygomaticoorbital artery, zygomaticotemporal artery, zygomaticofacial artery, and infraorbital artery. Fascial septae of midface (left to right): lateral cheek septum, medial cheek septum, middle cheek septum, and nasolabial septum. The orbicularis membrane is superior to them. Forehead vessels (left to right): horizontal limb of frontal branch of superficial temporal artery, ascending branches of supraorbital artery (emerging from below supraorbital ligament), supratrochlear artery, paracentral artery and angular artery, central artery and dorsal nasal artery, and central vein. This figure is an original creation by the first author, prepared for this publication.
This study unveils the contemporary microanatomy of the integumentary system. The ensuing sections delve into its many microsurgical implications.
Dissection Planes
Surgical planes are often collagen-rare, allowing blunt dissection. Fluid from tissue edema, fasciitis pathogens, and injections travel along these planes. Arteries traverse them, but they still require delicate handling to avoid bleeding.
The deep-dermal plane, running within the dermo-hypodermal interface, begs description. There is no clear interface, especially in distal extremities (Figure 1 label #10 and Table 2). Indeed, full-thickness skin grafts often contain elements of both layers [27,47]. Horizontal vessels populate this wide region of deep dermis and subdermis [18,21,24,27]. This network can be subdivided into the deep dermal (Figure 1 label #53) and subdermal plexi (Figure 1 label #54). Separation requires meticulous fat dissection or hydrodissection through the deepest reticular dermis [1,2,9]. We believe this plane divides the plexi unequally, favoring the subdermal plexus. Angiography reveals the insufficiency of the deep dermal plexus [1]. Some surgeons propose that vertical perforators run between the subdermal and deep-dermal plexi [2]. However, imaging reveals these two plexi are continuous, horizontal, and lack any intervening solid membrane [9,18,24]. The vertical vessels observed are likely plexus vessels displaced by hydrodissection or descending adipofascial branches of these plexi (Figure 1 label #55).
Tissue Viability, Engraftment, and Plexus-To-Plexus Apposition
Neovascularization begins around day 3 of tissue transplant [57,67]. It is robust enough to support most fasciocutaneous flaps by the 12th postoperative day [68]. The periphery of skin flaps derives perfusion from wound bed neo-vessels [57]. Vessels grow at a rate of approximately 0.2 mm per day, continuing up to distances of 2–5 mm [41]. More intervening fat or scar prevents neovascularization. These new vessels are quite small [69,70], and pedicle injury can compromise flaps even years after insetting [69,71]. This often complicates fatty abdominal flaps and muscular flaps. Both flaps have barriers to neovascularization (fat and perimysium). Skin flaps undergo revascularization faster than muscle flaps [72]. Skin flaps have large exposed plexi, enabling arteriogenesis (widening of pre-existing arteries) and angiogenesis (sprouting from existing arteries) before neovascularization (formation of vessels from progenitor cells). Their different perfusion patterns contribute to their differential angiogenesis; skin flaps have an initial vasoconstrictive phase after sympathetic denervation, leading to more hypoxia-signaling, promoting angiogenesis, whereas muscle flaps are less sensitive to denervation, and hypoxia increases perfusion along the pedicle [73]. Supercharging adipose tissue with a dermal plexus flap enhances its viability [74].
Early engraftment enhances the viability of grafts and thin flaps [2,3,4,70,75]. Full-thickness skin grafts (FTSG) may receive dermal plexus perfusion from their margins. Yet, they cannot survive over a poorly perfused bed wider than 12 mm [8,76–78]. Dermal plexus flaps also have a limited zone of perfusion (Table 1) [1], and similarly necrose over poorly perfused beds [6]. Adipose tissue is slippery; engraftment technique is important for composite graft take.
Apposition of the plexi in grafts/flaps and their wound beds improves outcomes [2,79–81]. It leads to inosculation (direct anastomosis) of pre-existing vessels, facilitating engraftment. Graft success may be related to dermal vascular density, which is greater in retroauricular, scalp, thigh, and plantar dermis as compared to the cheek, groin, peri-clavicular, back, and buttock dermis [8]. In the face, extensive communication between angiosomes diminishes the importance of engraftment. Indeed, whole-face transplants and large keystone flaps can be reliably perfused on a single perforator (Figure 2) [82]. Septae between adipocytes contain vessels (Figure 1 label #13). Including the septae in composite grafts thus improves viability [2,46]. Septae are well-defined in the groin, mastoid region, and other anchoring sites (Table 2) [11].
There are different techniques for preserving plexi during dissection. Partial debridement, retaining the deepest, flimsy layer of reticular dermis, maintains the bed’s deep dermal plexus (Figure 1 label #11) [2]. To maintain the subdermal plexus in a raised flap/graft, surgeons preserve at least 1 mm of fat during scalpel/scissor dissection [2,83], and 3–5 mm during open-tip liposuction and/or arthroscopic shaving [84,85]. During de-fatting, maintaining the honeycomb fascia helps preserve septal vessels (Figure 1 label #13) [2]. It is very difficult to completely de-fat a pure-skin perforator flap [1,49]. Defatting to less than 1 mm can insult plexi and deep dermal structures, compromising perfusion and contributing to post-inflammatory hyperpigmentation [2,86]. Preserving more than 4 mm of fat limits engraftment [2,87]. The suprafascial plexus is easily maintained by dissecting fat off it [79–81]. In patients with thick fascia, as seen in chronic lymphedema, deep subfascial plexi are made accessible by fascial thinning to 1 mm [75].
Neurotization
Skin neurotization causes vasospasm [72]. Skin sympathetic denervation enhances perfusion after a 24–48-hour vasoconstrictive phase [73]. This may support cutaneous flap perfusion. In contrast, muscle flap perfusion is regulated by metabolic demands [72].
Neurotized tissue can achieve near-normal skin sensitivity [47,88–91]. Flap debulking reduces the distance between skin and deeper nerves, improving sensory outcomes [79–81,91,92]. Though a subpapillary nerve plexus may exist, the majority of sensory innervation comes from the dermal nerve trees that ascend with microangiosome arteries [24,29]. Thus, hinged grafts/deep dermal plexus flaps are unlikely to be neurotized except through their bed.
Post-inflammatory Hyperpigmentation
Post-inflammatory hyperpigmentation (PIH) is a morbid complication of free tissue transfers. It is mediated by dermal fibroblasts and sebocytes, which are abundant in the dense reticular dermis (Figure 1 label #9,38) [93–96]. Cells are activated by ischemia, desiccation, and inflammation, resulting from mechanical trauma, inflammatory dermatoses, photodamage, and endogenous metabolic stresses [96–99]. This is the rationale for prophylactically prescribing oral antioxidants (vitamins C and E) and topical moisturizers [47]. Epithelial pigmentation is brown and fades over months. Dermal pigmentation is grey-brown and persists, particularly in dark-skinned people [100]. The ‘melanocyte-migration hypothesis’ for PIH states that inflammatory signals damage the basement membrane, precipitating melanocyte incontinence into the upper dermis [101,102]. This is readily observed in ‘pie-crusted’ skin grafts, which retain dyschromic stab-site scars [4]. Recent evidence challenges this, suggesting PIH results from activation of dormant subdermal melanoblasts [103].
Current microanatomical concepts and clinical observations reveal the complex etiopathogenesis of chronic PIH. While further analysis is necessary, this preliminary review offers the following insights into chronic PIH associated with split-thickness skin grafts (STSG), FTSG, skin-fat composite grafts (SFCG), and very thin flaps:
PIH is occasionally useful to enhance skin pigmentation, such as during flap debulking, although the extent of pigmentation remains unpredictable [79,125]. Exploring the potential of dermal substitutes to mitigate graft associated PIH could be pursued, particularly in dark-skinned populations [126].
Debulking
The ultimate aim of debulking surgery is to address both cosmetic concerns (contour, scarring, pigmentation, hirsutism) and functional issues (pliability, sensitivity, grip, skin quality, hindrance to wearing clothes/shoes, speech, swallowing, etc.) in a single stage. Radical debulking results in thin dermal-plexus flaps, which is suitable for tasks like degloved wound coverage, lymphedema debulking, and thick flap revision [3,4,75,127]. Excisional debulking and liposuction are common techniques used for debulking. Other techniques include liposuction with arthroscopic shaving, intricate tissue rearrangement, laser procedures, and coverage with regionally expanded tissue [127]. Among these options, liposuction is the least traumatic for the wound bed. However, debulking can be uneven and often requires multiple stages, and a layer of fat must be retained to control bleeding. Fibrosed fat resulting from inflammation or irradiation is challenging to remove using conventional suction methods. For this purpose, open-tip liposuction and arthroscopic shaving are effective, although they come with the risk of pedicle injury [85,127,128]. Liposuction in combination with circumferential tissue rearrangement can be employed for debulking turnover flaps [129,130].
Debulked flaps are usually perfused by their pedicles and the vessels in the wound bed [57,69,131]. The revascularization of the flap involves early proximal flap arteriogenesis and late distal flap angiogenesis [69]. Often, wound bed angiogenesis alone may prove inadequate [68]. To enhance early perfusion from the surrounding skin, the design may incorporate beveled or de-epithelized wound margins. This design promotes the alignment of skin structures, facilitating inosculation between the remaining vessels of the deep dermal and subdermal plexi [45–47,132].
Head & Neck Melanoma Metastasis
Head and neck melanomas have a 22% higher mortality compared to other regions, suggesting regional differences in anatomy and melanoma behavior [133]. Lymphatics are concentrated in the scalp and lateral neck. Node biopsies in these regions have a higher detection rate than those of the face and ear [134]. However, detection by lymphoscintigraphy and sentinel lymph node biopsy shows poor efficiency in this region compared to others [135]. Low node positivity is a characteristic specific to melanomas with a diameter of >2.0 mm [52,136]. These findings suggest that large head and neck melanomas also metastasize hematogenously [52,136]. Considering the low prognostic value of sentinel node dissection, early surveillance using 3D SPECT/CT (three-dimensional single photon emission computed tomography/computed tomography) or empirical adjuvant therapy might be considered in the future [52,136]. The challenges of operating in this region could impact outcomes.
Injection Depth
The size of injected particles influences cohesivity, fluency, and degradation time. This guides decisions about appropriate injection site and depth [137]. The different densities of dermal collagen layers also impact fluid flow [9]. The thick middle dermis is dense and reflective, dividing fluids injected above and below it.
Superficial dermal injection is performed at angles up to 12 degrees. It requires little pressure, readily forming a wheal and can be confirmed by visualizing the needle outline through ‘tenting’ [138]. It is useful for hydrodissection before partial debridement [39], or for superficial hypoperfusion using epinephrine-local anesthesia for hair transplant, as per our experience. To fill small wrinkles, less-cohesive fillers (e.g., Belotero hyaluronic acid) are injected in this plane, as they are moldable and spread evenly [139]. Microfat grafts are also injected into the superficial dermis by injecting while withdrawing the needle from pinched-up skin, minimizing the risk of dermal vascular injection and fat embolism [140]. High-cohesivity fillers (e.g., Radiesse calcium hydroxyapatite and Bellafill polymethylmethacrylate) are not used superficially as they form palpable nodules [141]. Using smaller needles at an angle reduces the risk of them backtracking into the superficial plane [141].
Middle dermal injection is painful and requires high pressure. It is used in scalp infiltration with epinephrine-local anesthetic, with the rationale that deeper infiltration tends to track into the loose areolar plane, minimizing the local effect [142].
Deeper injections remain below this dense middle dermis and tumesce the deep-dermal/subcutaneous plane. Epinephrine-local anesthetic infiltrated into this region may constrict the stem of microangiosomes, regulating flow to the more superficial layers [21,40]. Classical subdermal large-diameter fat grafts are relatively under-perfused and are complicated by atrophy, cysts, nodules, and necrosis [140]. Deeper wrinkles are broken by needle subincision and volumized by high-cohesivity fillers [139].
Resolving Surgical ‘Paradoxes’
Reviewing microanatomy clarifies seemingly conflicting surgical observations. Engrafting dermal plexus grafts and flaps and improving marginal perfusion through the alignment of beveled dermal surfaces may enhance tissue viability and patient outcomes.
Regarding dermal plexus flaps, which are mostly fat-free, the deep dermal plexus supplies up to about 12 mm from the flap base [1,8,76–78]. Consequently, large dermal plexus flaps are predominantly perfused through engraftment [3–5]. Poor engraftment compromises debulked dermal plexus flaps [6,7]. Treating these flaps as hinged grafts, such as when redraping limbs, proves to be a successful approach [3–5].
Aligning vessels of similar sizes leads to early anastomoses (inosculation). This principle is exploited in plexus-to-plexus apposition techniques, in which plexi from donor and recipient site tissues are approximated. Inosculation is also promoted by increasing the area of marginal plexus-to-plexus apposition, as accomplished in beveled-margin grafts and flaps [45–47,132]. This latter technique is particularly effective in composite grafts from retroauricular skin, as this skin receives predominantly marginal perfusion, and thus, possesses well-developed deep dermal and subdermal plexi [8]. The ability to survive over poorly perfused beds and to be revascularized by inosculation within 24 hours blurs the distinction between beveled-margin retroauricular composite grafts and free flaps. This holds especially true considering both undergo an early vasoconstrictive phase due to sympathetic denervation [73].
Microanatomical concepts must inform surgical practice. Understanding dissection planes helps preserve vascular plexi and ensure tissue viability. The relationships between perforators, angiosomes, and surrounding neurovasculature guide flap design. Understanding the limits of microangiosomes and the deep dermal plexus, as well as the absence of the superficial dermal plexus, underscores the significance of engrafting thin primary and debulked flaps. Apposition of plexi within donor and recipient tissues can enhance tissue viability and neurotization. Chronic post-inflammatory hyperpigmentation after free tissue transfer seems to be influenced by tissue ischemia, donor tissue melanocyte density, recipient site trauma, and dermal fibroblast density. Techniques to enhance microsurgical safety are discussed.
Received date: June 04, 2023
Accepted date: July 21, 2023
Published date: September 14, 2023
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
This research was funded in part through National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2023 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
Division of the lateral plantar artery does not jeopardize the foot circulation because of anastomosis of the lateral plantar artery with the dorsalis pedis artery at the first intermetatarsal space. However, care should be taken with patients with peripheral artery occlusive disease and the flow of dorsalis pedis artery should be confirmed before surgery. Given the advantages of sizable vessel, easy dissection, and proximity to the defect, we believe that the lateral plantar artery might be a valuable option as recipient vessel for lateral plantar forefoot reconstruction.
Health care systems in many countries are confronted with increasing economic limitations. Thus, complex microsurgical procedures and extensive rehabilitation programs are poorly compensated. However, this case demonstrates a dramatic reduction of socioeconomic expenses by allowing a potential radiocarpal amputee to return to work for another estimated 30 years.
Pedicled anterolateral thigh flap is a versatile option for reconstruction of complex soft tissue defects in varied anatomical regions. Its wide arc of rotation and less donor site morbidity are its added advantages.
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
Supraclavicular flap is an excellent fasciocutaneous flap for head and neck reconstruction due to its close color and texture match. In general, long flaps are required, but with the risk of distal necrosis. The aim of this study is to assess the relationship between the length and distal end necrosis of the supraclavicular flap.
The authors describe various patient and breast-related factors that influence surgical outcomes while also addressing some techniques and principles for aesthetic microsurgical reconstruction.
The authors present a retrospective review of 7 patients who underwent wide excision of the malignant tumors around the clavicle. Patient demographics, clinical details, the arc of rotation, outcome, and complications were analyzed.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The senior author (Dr. Isao Koshima) designed a tibial osseo-periosteal (TOP) flap. TOP flap has a favorable anatomical position with a thin skin around it, hence it is a good option for an island flap. TOP flap can be used for various mild to moderately sized osteo-cutaneous defects with low morbidity. In this article, the authors describe their experience of the first reported cohort of TOP flaps in clinical practice.
The authors present a revised application of the composite iliac crest bone free flap for hemimaxillectomy defects. This flap solely can give a support to the eye globe and the dental implants without osteotomies and titanium meshes. The upper ridge of the flap can be shaped to replicate the curvature of the orbital floor. The inner oblique muscle and the deep fascia can serve as an additional material for separation of the oral cavity from the nasal airway. Utilization of the Customized Contour Implants gives a surgeon a refining instrument for aesthetic correction of facial projection.
Patients with gynecological abdominal wall malignancies can benefit significantly from radical resection and autologous reconstruction. The pedicled anterolateral thigh flap is the preferred donor site, offering a reliable solution to abdominal wall reconstruction in this setting. The satisfactory results should prompt a more aggressive surgical approach for these patients. This article describes the authors' experiences with the abdominal reconstruction following surgical resection of gynecological abdominal wall malignancy using pedicled anterolateral thigh flap.
The ALT and AMT flaps are the most commonly used thigh free flaps for intraoral reconstruction. Recently, PAP flap has been proposed as an alternative. This study aimed to compare the thickness of these thigh flaps and to identify the factors influencing flap thickness in our population.
A thin profunda artery perforator flap harvested from the left thigh is shown in this video. Preoperative computed tomographic angiography is used to assess morphology of the perforators and its branches, pedicle length and vertical location of the two branches from the ischial tuberosity. These measurements are translated on to the patient. Locations of both branches are confirmed with a handheld doppler. The authors concluded that preoperative computed tomographic angiography is a useful technique to provide detailed anatomic information on morphology of perforators, course through the septum or muscle above the deep fascia and skin thickness. Computed tomographic angiography allows quick and easy assessment of the whole vascular anatomy of the leg and helps to arrive at the decision about selection of the best flaps based on the characteristics of the defect and on the individual anatomy of the patient.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
Smartphones and cellular technology have revolutionized flap monitoring. Smartphones provide low-cost thermal imaging alternatives to flap monitoring. It remains unclear, however, whether this method is accurate or reliable. It may be challenging to use smartphone thermal imaging when clinicians fail to communicate clinically relevant events. The authors demonstrate three instances in which the thermal imaging information on smartphones was misleading. Each case is analyzed to determine how clinical decisions should be made.
The likelihood of donor site ischemia following the harvesting of a fibula flap is extremely low, but it is potentially lethal if it occurs. The authors describe a case of ischemia of the lower extremity following a free fibula harvest for head and neck reconstruction. The authors discuss preoperative, intraoperative, and postoperative strategies to assist in diagnosing and managing risks associated with free fibula flap harvesting in this paper.
The article describes the case of a 27-year-old man who suffered from scarring at the penopubic junction as a result of penis lengthening surgery. The authors explain how they repaired the penopubic junction by using a pedicled superficial circumflex iliac artery perforator flap. The authors also propose a safe and effective hybrid technique for harvesting superficial circumflex iliac artery perforator flaps. According to the authors, the pedicled superficial circumflex iliac artery perforator flap is an effective method for reconstructing the penopubic scar.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This study introduces an advanced tubularized radial artery forearm flap (RAFF) technique, marking an enhancement over traditional methods in addressing complex nasal reconstructions. It integrates functional and aesthetic considerations through a structured, multi-stage reconstruction process, emphasizing the use of tubularized flaps. Key learning points include the detailed crafting of stable nasal passages, strategic use of costal cartilage for robust structural support, and tailored postoperative care with silicone splints. The tubularized RAFF technique not only optimizes patient outcomes and quality of life but also provides plastic surgeons with critical insights to refine their techniques in facial reconstruction. Indispensable for professionals in the field, this article enriches the understanding of sophisticated reconstructive challenges and solutions.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This manuscript showcases an advanced surgical approach for treating malignant giant cell tumor of bone, emphasizing precision and ethical considerations. It leverages innovative pedicled flap technologies, as opposed to free flaps, enhancing limb functionality and patient quality of life. This technique equips surgeons with evidence that tailored surgical strategies can significantly improve outcomes in complex cases. The paper discusses technical challenges and highlights the application of supercharging and superdrainage techniques in limb reconstructions, methods well-established in microsurgery but infrequently used in oncological contexts. These techniques are crucial for optimizing flap viability and ensuring surgical success. Additionally, the manuscript underscores the profound impact of these advancements on patient lives, offering hope and showcasing tangible benefits. This narrative, blending scientific analysis with patient stories, enriches the understanding of limb reconstruction innovations in oncological surgery, making it invaluable for surgeons.
This systematic review and meta-analysis provide a pragmatic evaluation of drain-free versus drain-based DIEP flap techniques for breast reconstruction, challenging the traditional reliance on drainage. By analyzing postoperative outcomes, the study highlights the potential for refining surgical strategies to enhance patient comfort and recovery without compromising safety. The findings offer a neutral perspective, suggesting that clinical practice may not necessarily depend on the use of drains. This revelation prompts medical professionals to reassess existing surgical approaches and may catalyze a paradigm shift in postoperative care. Presented with clear narrative and rigorous data analysis, the article encourages readers to consider the broader implications of surgical innovations on patient care protocols.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
This case report describes authors successfully attempt to perform a topographically correct sural nerve transplantation in the extracranial facial nerve stem using the intraneural facial nerve stem topography proposed by Meissl in 1979. This is the first reported case of successful fascicular nerve grafting of the facial nerve stem following extensive laceration.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
This captivating article explores a diverse range of pivotal subjects within the realm of microsurgery, including microangiosomes, dissection planes, and regionally variable clinically significant structures. By critically examining and expanding upon these concepts, the study not only pushes the boundaries of our knowledge but also challenges established practices in the field. The comprehensive investigation into the viability and potential engraftment of dermal plexus flaps, along with meticulous analysis of head and neck perfusion and lymphatic drainage, reveals intricate nuances that shed light on the anatomical intricacies of these vital regions. These groundbreaking findings, supported by meticulous illustrations, provide an invaluable resource for understanding dissection planes, flap viability, neurotization, hyperpigmentation, tissue engraftment, and debulking techniques in fasciocutaneous microanatomy, thus facilitating their secure implementation in microsurgery. While the article holds immense value for the scientific community and merits serious consideration for publication, addressing minor concerns beforehand will ensure the highest standards of accuracy and clarity.
This article delves into the intricate microanatomy of the integumentary system, placing a strong emphasis on the importance of informed surgical practice and maintaining tissue viability. By addressing misconceptions and providing updated insights, it sheds light on the nuances of cutaneous microvasculature, with a particular focus on the regional variabilities in connective and adipose tissue, venolymphatic and neuroarterial systems, and specialized organs. The discussion further explores the implications of tissue engraftment, plexus-to-plexus apposition, neurotization, and post-inflammatory hyperpigmentation on surgical outcomes. Through this comprehensive analysis, the article significantly contributes to the understanding of microsurgery, enabling the enhancement of surgical techniques and ultimately improving patient care. Given its thorough review and clinical relevance, the publication of this article, following the resolution of specific concerns, is highly justified.
This comprehensive review article offers a profound and insightful analysis of several pivotal subjects within microsurgery. The authors challenge existing knowledge in microsurgery by meticulously examining microangiosomes, dissection planes, and various structures' clinical significance. Notably, the article extensively explores the viability and potential grafting applications of dermal plexus flaps, providing a comparative analysis between subdermal and deep-dermal plexi. Augmented by detailed illustrations throughout the review, readers gain a comprehensive understanding of the intricate microanatomy of the skin and underlying tissues, enabling the practical integration of these invaluable insights in the field of microsurgery. In essence, this paper has the potential to propel the field of microsurgery forward and thus merits sincere consideration for publication. However, it is essential to address a few minor issues to ensure utmost precision before its release.
Bajwa MS, Afzal MO, Hussain A, Farooq UK, Bashir MM, Shahzad F. Redefining fasciocutaneous microanatomy: An illustrated review of current concepts and their clinical correlates. Int Microsurg J 2023;7(1):2. https://doi.org/10.24983/scitemed.imj.2023.00174