Objective: Refractory chylous ascites in systemic lupus erythematosus (SLE) is a rare and challenging condition with limited management guidance. Most published reports are isolated cases, offering minimal insight into its clinical profile. In the absence of established surgical strategies when conservative treatments fail, this work provides the first comprehensive review of SLE-associated refractory chylous ascites to establish an evidence-based therapeutic framework. It also documents the first successful application of a lymphatic bypass procedure in this context.
Methods: A 36-year-old man with SLE developed refractory chylous ascites after a 13-year disease course. Prior treatments, including immunosuppressive therapy, vascular embolization, and laparoscopic surgery, failed to control fluid accumulation. The patient subsequently underwent an innovative microsurgical procedure that combined a deep inferior epigastric lymphatic cable flap (DIE-LCF) with a gastroepiploic vascularized lymph node flap (GE-VLN) to establish an alternative lymphatic drainage route, referred to as the DIE-GE lymphatic bypass. To clarify optimal management strategies for this rare complication, a comprehensive review of 19 cases was conducted, including the present case.
Results: The patient achieved complete resolution of chylous ascites within one week and was discharged on postoperative day 10. No recurrence, flap ischemia, or abdominal wall herniation was observed throughout the one-year follow-up period. Literature review demonstrated a predominance of female patients (84%), with a median age of 38 years. Chylothorax was reported in 79% of cases. Corticosteroids were administered in 95% of patients, often in combination with immunomodulatory agents. Among the 11 patients treated with medical therapy alone, primarily corticosteroids and other immunosuppressants, only 2 achieved complete remission, corresponding to a success rate of 18%. This modest efficacy underscores the limitations of pharmacologic therapy in addressing the multifactorial pathophysiology of SLE-associated chylous ascites. Of the 19 cases reviewed, 8 patients received additional surgical interventions. Among the 7 who underwent conventional procedures such as paracentesis, peritoneovenous shunting, or thoracic duct repair, only 3 achieved complete remission, yielding a 43% success rate. In contrast, the present case demonstrated complete and sustained remission following DIE-GE lymphatic bypass. This outcome raised the overall complete remission rate among surgically managed patients to 50% (4 of 8). The favorable response observed suggests that DIE-GE lymphatic bypass may represent a promising microsurgical option when both standard immunosuppression and conventional surgical approaches prove inadequate.
Conclusion: This study introduces the DIE-GE lymphatic bypass, a novel microsurgical technique for reconstructing lymphatic drainage. Its use is supported by a successfully treated case and a comprehensive literature review. The approach achieved both rapid resolution of ascites and sustained anatomical stability, providing preliminary evidence of therapeutic potential and a foundation for future clinical adoption.
Chylous ascites is characterized by the accumulation of triglyceride-rich, milky fluid in the peritoneal cavity. This fluid contains high concentrations of proteins, immunoglobulins, and essential nutrients. When present in large volumes, it can lead to immunosuppression, malnutrition, and electrolyte imbalances. The condition most commonly arises from obstruction or disruption of lymphatic drainage. Common causes include trauma, surgery, malignancy, infection, cirrhosis, and congenital lymphatic anomalies. In adults, malignancy is the leading etiology, followed by postoperative complications, inflammatory disorders, and infections. Mortality rates vary depending on the underlying causes, ranging from 40% to 70% [1–3].
Therapeutic Limits in SLE Chylous Ascites
Chylous ascites associated with systemic lupus erythematosus (SLE) is exceptionally rare and poses significant challenges in therapeutic management. Most published reports are limited to isolated cases, offering minimal insight into the clinical course, treatment response, and long-term prognosis. Conservative management generally includes immunosuppressive therapy, parenteral nutrition, and repeated paracentesis. These interventions aim to reduce lymphatic leakage and preserve nutritional status. However, such strategies frequently fail to achieve durable disease control. Although surgical intervention may be considered in refractory cases, its indications and clinical efficacy remain inadequately defined. These limitations highlight the need for more robust, evidence-based therapeutic approaches.
Lymphatic Bypass in SLE Chylous Ascites
Given the limited efficacy of conventional therapies, microsurgical reconstruction has emerged as a potential strategy to reestablish lymphatic drainage in refractory cases. Ciudad et al. [4] previously described a novel approach combining a deep inferior epigastric lymphatic cable flap (DIE-LCF) with a gastroepiploic vascularized lymph node flap (GE-VLN), forming an alternative route for chylous fluid diversion. For clarity, we refer to this technique as the DIE-GE lymphatic bypass. It demonstrated clinical success in two non-SLE patients, one with refractory chylous ascites following kidney transplantation and another with lymphatic injury secondary to oncologic treatment. However, the original procedure left the fascial opening unclosed, raising concerns about postoperative herniation. To date, this technique has not been applied to cases involving autoimmune-mediated lymphatic inflammation, and its feasibility, safety, and required technical modifications in this context remain unestablished.
Novel Strategy and Literature Integration
We report the first successful application of the DIE-GE lymphatic bypass in a 36-year-old male with SLE-associated refractory chylous ascites unresponsive to conventional treatment modalities. This case offers preliminary clinical evidence supporting the feasibility of this approach under autoimmune-inflammatory conditions. However, a single case alone cannot resolve persistent uncertainties in surgical decision-making, particularly when conservative strategies fail. Existing literature remains fragmented, comprising primarily isolated case reports without comparative synthesis. To address this knowledge gap, we conducted a critical appraisal of previously reported cases and present the first comparative analysis focused on SLE-associated chylous ascites.
Study Aim
This study aims to address the current lack of evidence by introducing a viable and innovative surgical alternative for patients with SLE-associated refractory chylous ascites. It seeks to establish preliminary clinical support for the DIE-GE lymphatic bypass as a therapeutic option. Such foundational evidence may inform future case series and support the development of standardized surgical protocols for this rare clinical entity.
A 36-year-old male presented with refractory chylous ascites. He had a 13-year history of lupus nephritis associated with SLE. A multidisciplinary team conducted a comprehensive evaluation and confirmed an etiological association between the ascites and underlying SLE. Despite multiple immunosuppressive regimens, supportive care, and conventional surgical interventions, the chylous fluid persisted. Following the failure of standard therapies, the patient was referred to the Department of Plastic and Reconstructive Surgery for further management.
Refractory Clinical Course Prior to Referral
Five months before referral, the patient underwent thoracotomy with ligation of a lymphatic leak to treat chylothorax, followed by pleurodesis. This procedure successfully resolved the pleural effusion. However, he subsequently developed progressive abdominal distension, which led to the diagnosis of chylous ascites. Conservative management comprising immunosuppressive therapy, serial paracenteses, a medium-chain triglyceride-based diet, and total parenteral nutrition failed to achieve clinical improvement.
Six weeks later, conventional lymphangiography with glue embolization was performed at the level of the fourth lumbar vertebra. Follow-up imaging conducted two weeks afterward confirmed persistent chyle leakage. Subsequent magnetic resonance lymphangiography was nondiagnostic, as no contrast uptake was observed following injection into the regional lymph nodes.
Two months before the DIE-GE lymphatic bypass, the patient underwent laparoscopic ligation of the identified chylous leak along the parietal peritoneum. The procedure failed to resolve the ascites. Following this unsuccessful intervention, he received supplemental albumin and additional supportive care. Ongoing fluid accumulation prompted referral to the plastic and reconstructive surgery team for evaluation of surgical candidacy for lymphatic bypass reconstruction.
Surgical Technique and Procedure
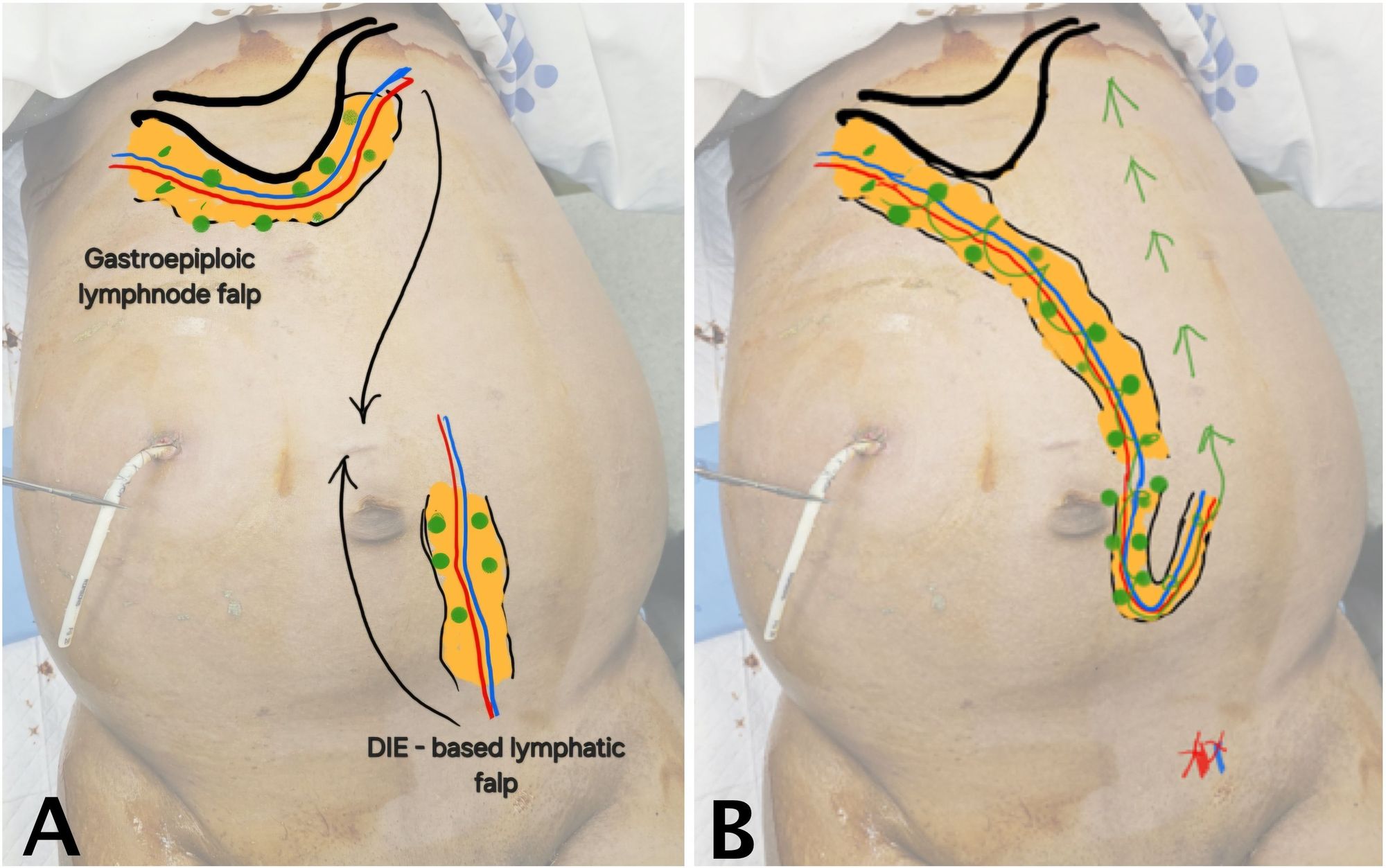
Due to persistent ascites despite multiple prior interventions, the patient underwent surgical management with the DIE-GE lymphatic bypass under general anesthesia. This procedure was designed to establish an alternative route for chylous fluid drainage by connecting two autologous, lymphatic-rich flaps. The overall surgical concept is illustrated in Figure 1.
As shown in Figure 1A, the recipient flap (GE-VLN) was harvested from the greater curvature of the stomach and oriented cranially. The donor flap (DIE-LCF), based on the deep inferior epigastric vessels and containing lymphatic cable tissue, was harvested from the lower abdomen and oriented caudally. After both flaps were elevated, microvascular anastomosis was performed to create a continuous lymphatic bypass pathway between them (Figure 1B). The technical aspects of flap harvest and preparation are outlined below.
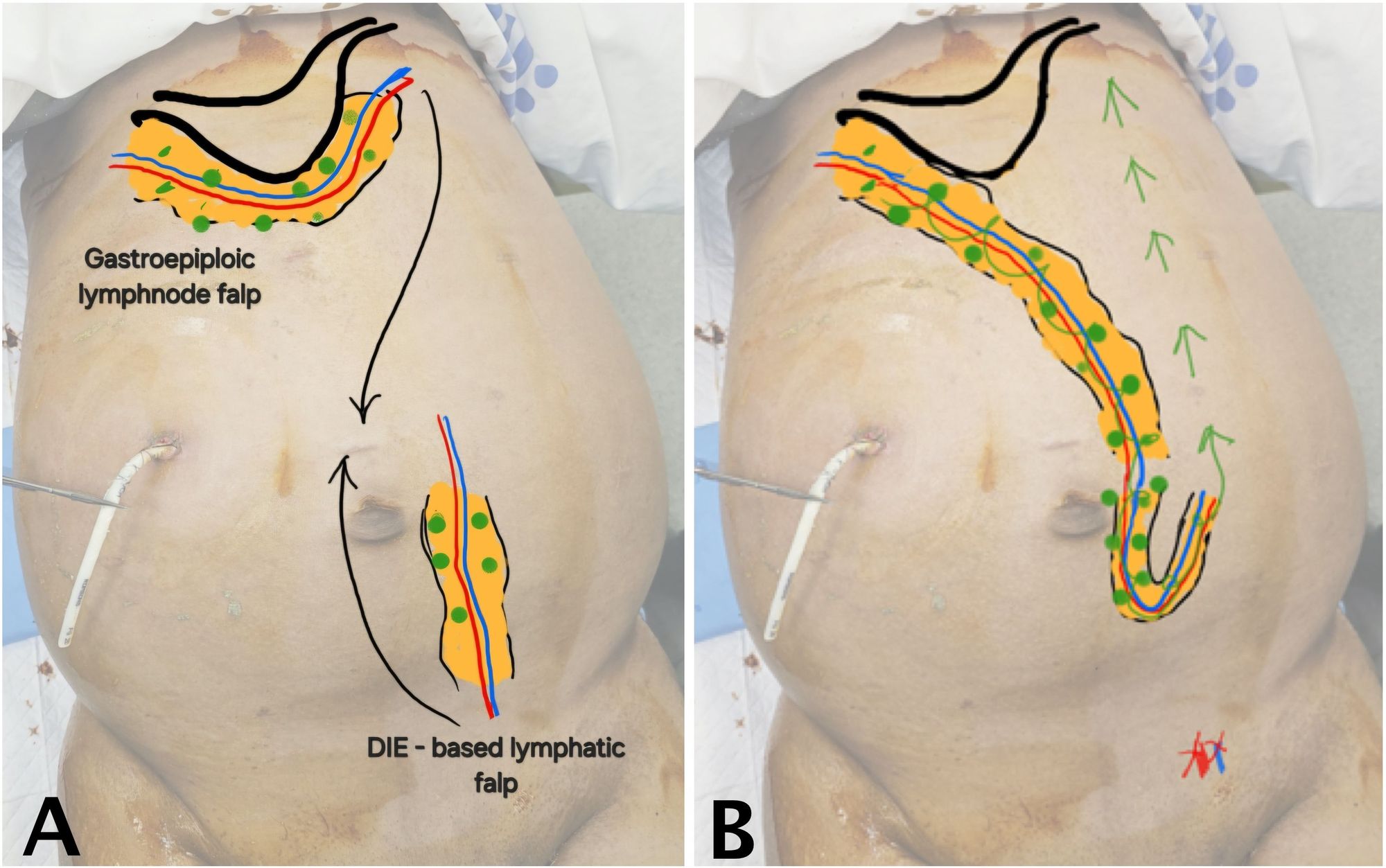
Figure 1. Schematic of DIE-GE bypass for chylous ascites. (A) Preoperative image showing the cranial GE-VLN harvested from the greater curvature of the stomach and the caudal DIE-LCF harvested from the lower abdomen, both depicted in orange. Green circles indicate lymph nodes; blue and red lines represent lymphatic channels and vascular pedicles. Arrows show the planned direction of flap transfer. (B) Postoperative image showing anastomosis of the two flaps to establish a bypass route for chylous fluid drainage into systemic circulation (green arrow). The red X marks the ligation site of the distal deep inferior epigastric vessels near the bifurcation of the external iliac vessels. Abbreviations: DIE-GE, combined deep inferior epigastric and gastroepiploic; DIE-LCF, deep inferior epigastric lymphatic cable flap; GE-VLN, gastroepiploic vascularized lymph node flap.
Harvesting of DIE-LCF and GE-VLN flaps
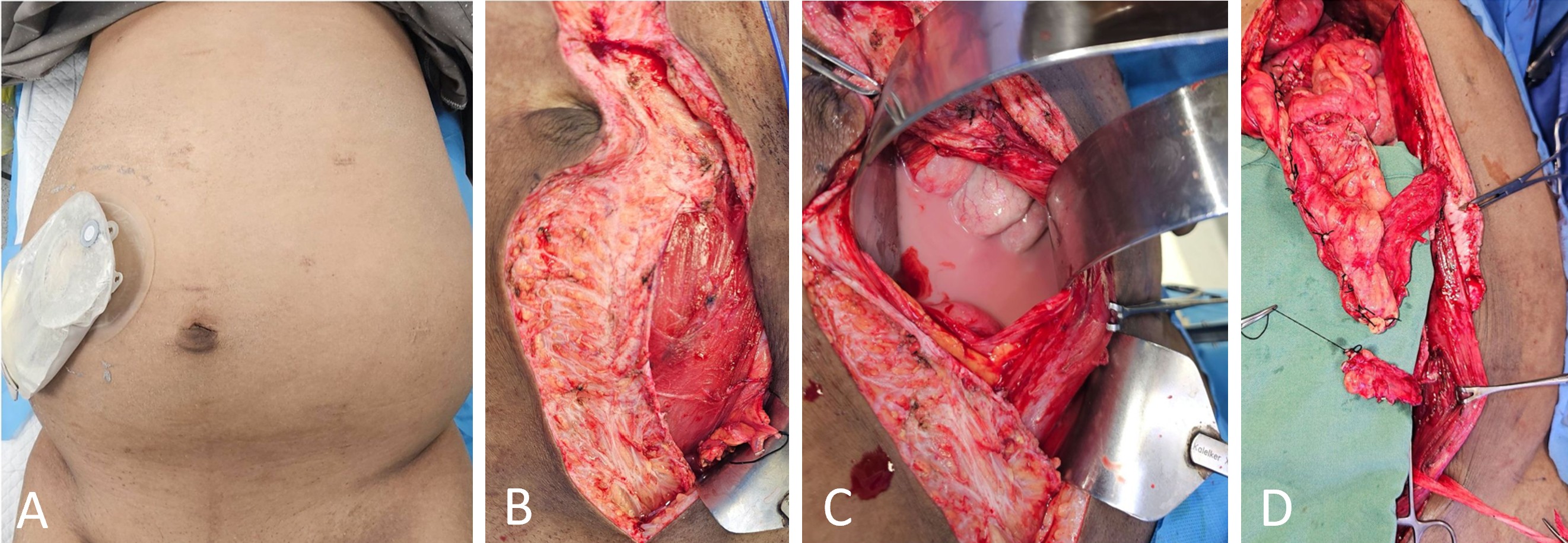
The flap-harvesting procedure is illustrated in Figure 2. Following a midline abdominal incision, the left rectus sheath was opened below the umbilicus. The rectus abdominis muscle was retracted to expose the deep inferior epigastric vessels, which were carefully dissected and mobilized. Adjacent adipofascial tissue containing lymph nodes and lymphatic channels was isolated with precision. The deep inferior epigastric vessels were ligated and transected near their origin at the external iliac vessels. The adipofascial tissue was then elevated on its vascular pedicle to form the DIE-LCF flap (Figure 2B).
Next, the rectus abdominis midline was opened to access the peritoneal cavity, allowing evacuation of approximately 4,000 mL of chylous fluid (Figure 2C). Dissection proceeded along the greater curvature of the stomach, where the left gastroepiploic vessels were ligated near the spleen. A GE-VLN flap was harvested based on the right gastroepiploic vessels (Figure 2D). To ensure adequate inclusion of lymph nodes and associated lymphatic channels, the flap was transected at least 5 cm distal to the vascular pedicle.
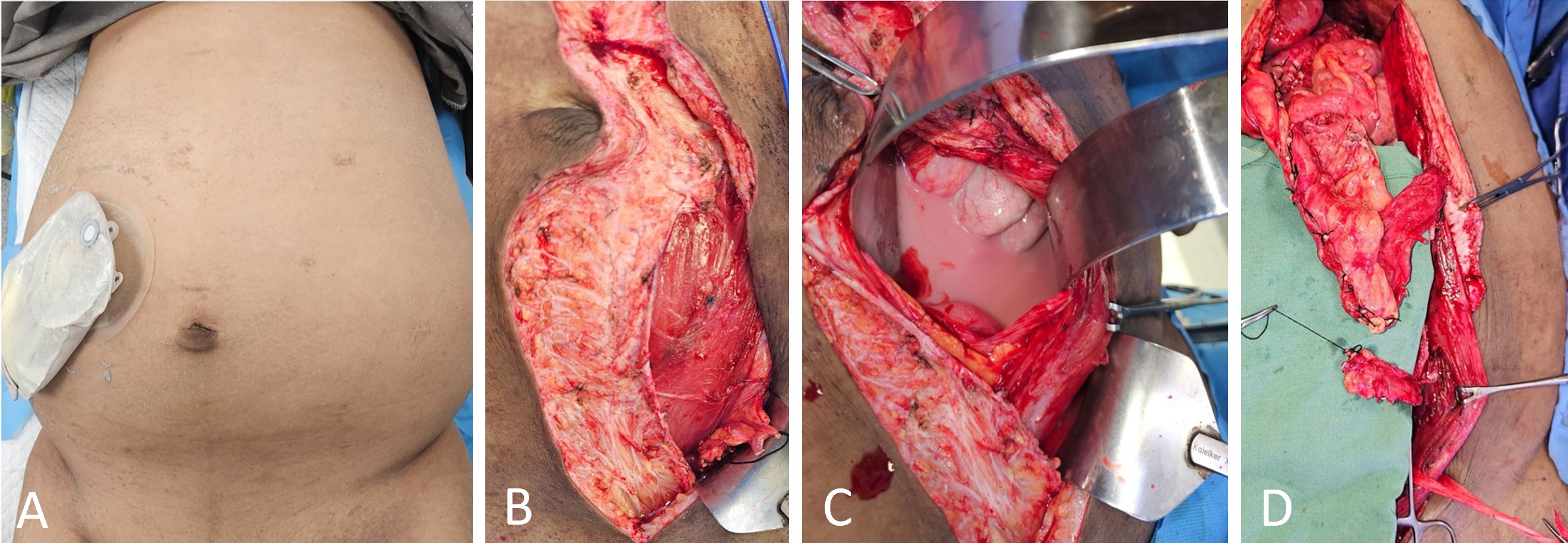
Figure 2. Operative findings and sequential harvest of lymphatic flaps for DIE-GE bypass reconstruction. (A) Preoperative image showing abdominal distension and placement of a drainage catheter for continuous evacuation of chylous fluid. (B) Intraoperative image showing harvest of the DIE-LCF along the lateral border of the left rectus abdominis. (C) Intraoperative image revealing milky chylous fluid accumulated within the peritoneal cavity. (D) Image after harvest showing the cranial GE-VLN and the caudal DIE-LCF prepared for transfer. Abbreviations: DIE-GE, combined deep inferior epigastric and gastroepiploic; DIE-LCF, deep inferior epigastric lymphatic cable flap; GE-VLN, gastroepiploic vascularized lymph node flap.
Vascular anastomosis and flap inset
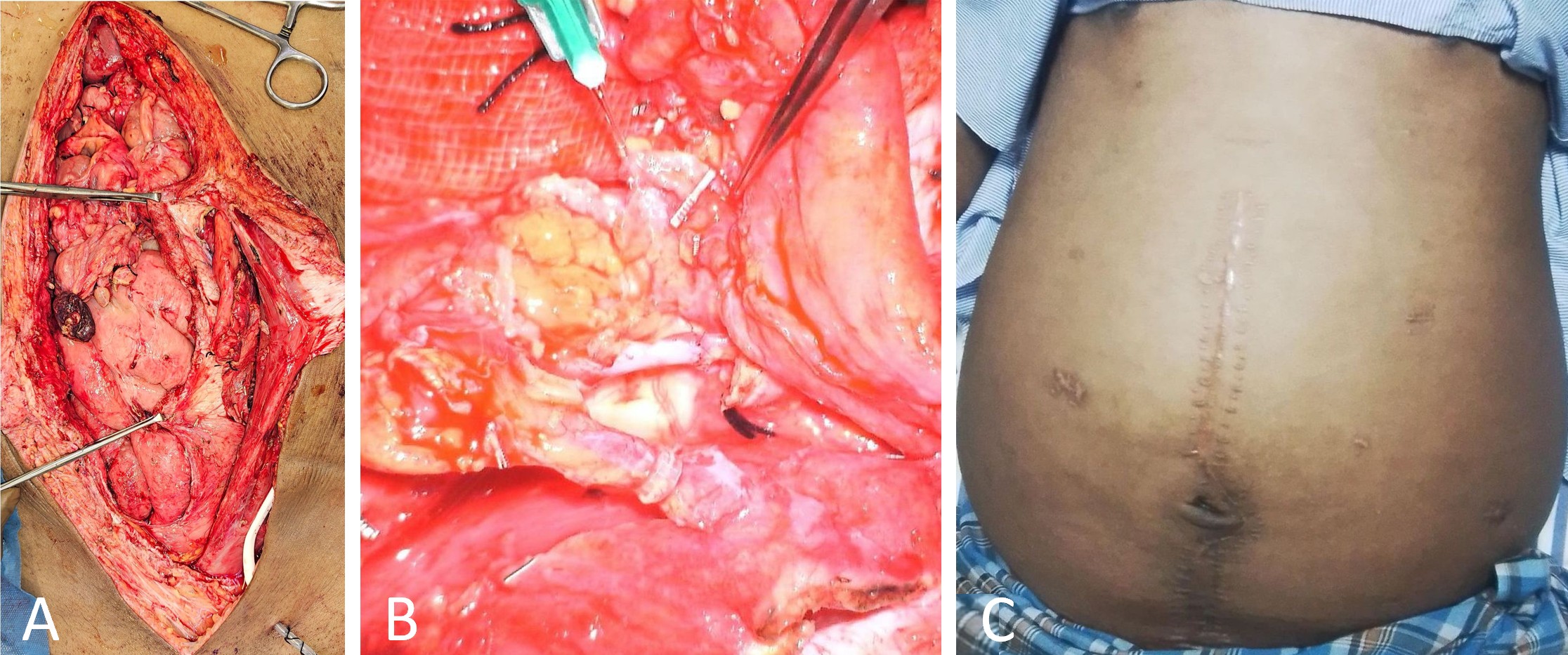
Following flap harvest, the GE-VLN was confirmed to provide sufficient pedicle length to reach the DIE-LCF without undue tension. It was introduced into the retrorectus plane through a cruciate incision in the left posterior rectus sheath. The DIE-LCF was rotated cranially to approximate the vascular pedicles (Figure 3A). Arterial anastomosis between the deep inferior epigastric artery and the left gastroepiploic artery was performed using 9-0 nylon sutures. Venous anastomosis was subsequently completed with a 2 mm coupler device (Figure 3B).
The distal ends of both flaps were loosely secured to reduce the risk of vascular kinking. To further prevent postoperative herniation, the flap was anchored to the parietal peritoneum within the abdominal cavity. Surgical drains were placed at the DIE-LCF pedicle site and within the peritoneal cavity. The rectus sheath was closed using 1-0 nylon sutures, followed by meticulous layered skin closure.
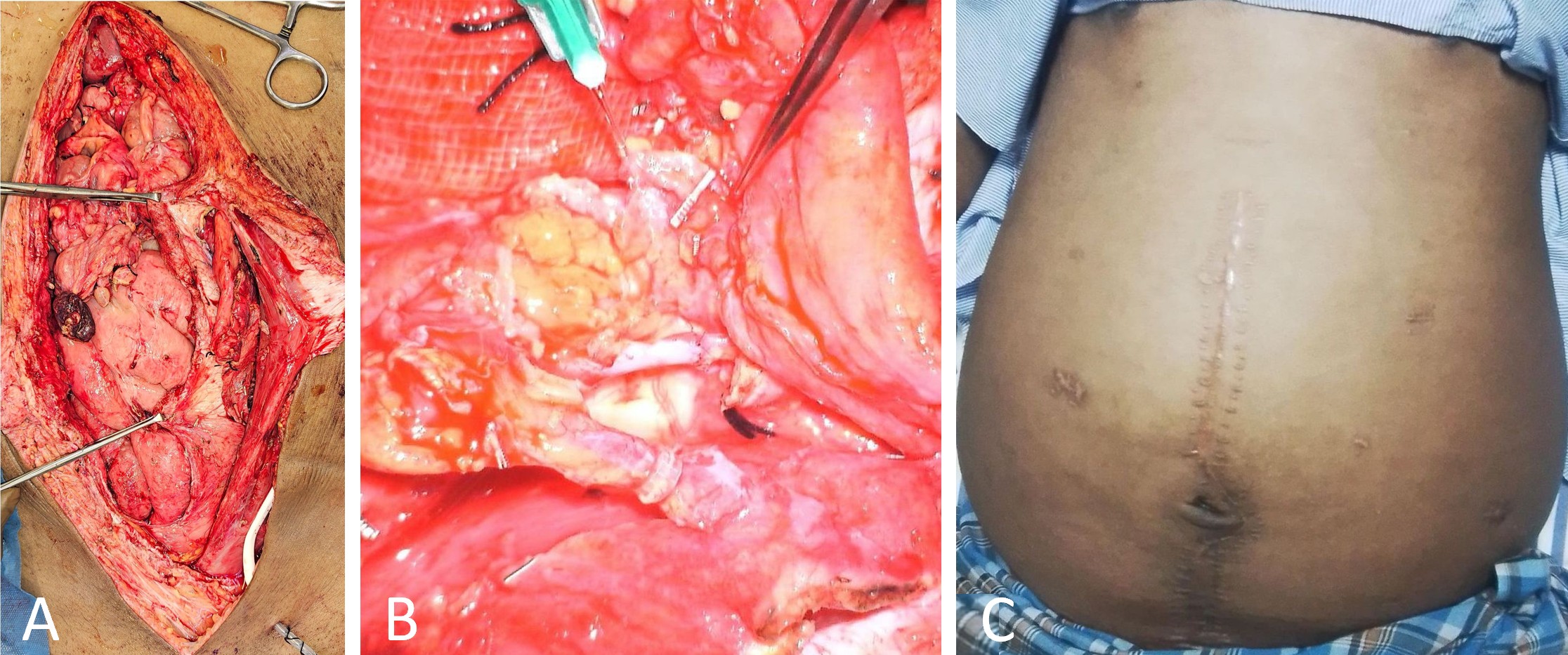
Figure 3. Intraoperative anastomosis and postoperative findings of DIE-GE bypass. (A) Intraoperative image showing the left posterior rectus plane before vascular anastomosis, with the cranial GE-VLN and caudal DIE-LCF positioned for connection. (B) Intraoperative close-up view showing the anastomosis sites, including arterial anastomosis (cranial) and venous anastomosis (caudal) completed with a coupler, both clearly visualized. (C) Postoperative image at one year showing reduced abdominal girth, absence of drainage catheters, and a visible midline scar. Abbreviations: DIE-GE, combined deep inferior epigastric and gastroepiploic; DIE-LCF, deep inferior epigastric lymphatic cable flap; GE-VLN, gastroepiploic vascularized lymph node flap.
Postoperative Care and Follow-up
Postoperative management was coordinated by a multidisciplinary team including specialists in critical care, clinical nutrition, gastrointestinal surgery, and plastic surgery. During the first postoperative week, the volume of chylous ascites progressively declined, permitting timely removal of surgical drains. The patient resumed oral intake without difficulty and was discharged in stable condition on postoperative day 10.
Following discharge, the patient maintained a high-protein diet and consistently used compression stockings, resulting in gradual resolution of lower limb and scrotal edema. At six months postoperatively, he was hospitalized for severe pneumonia at a local facility. During this admission, two episodes of localized intra-abdominal fluid accumulation were identified and drained. Biochemical analysis revealed serous, nonchylous fluid with negative triglyceride levels, confirming the absence of recurrent chylous ascites.
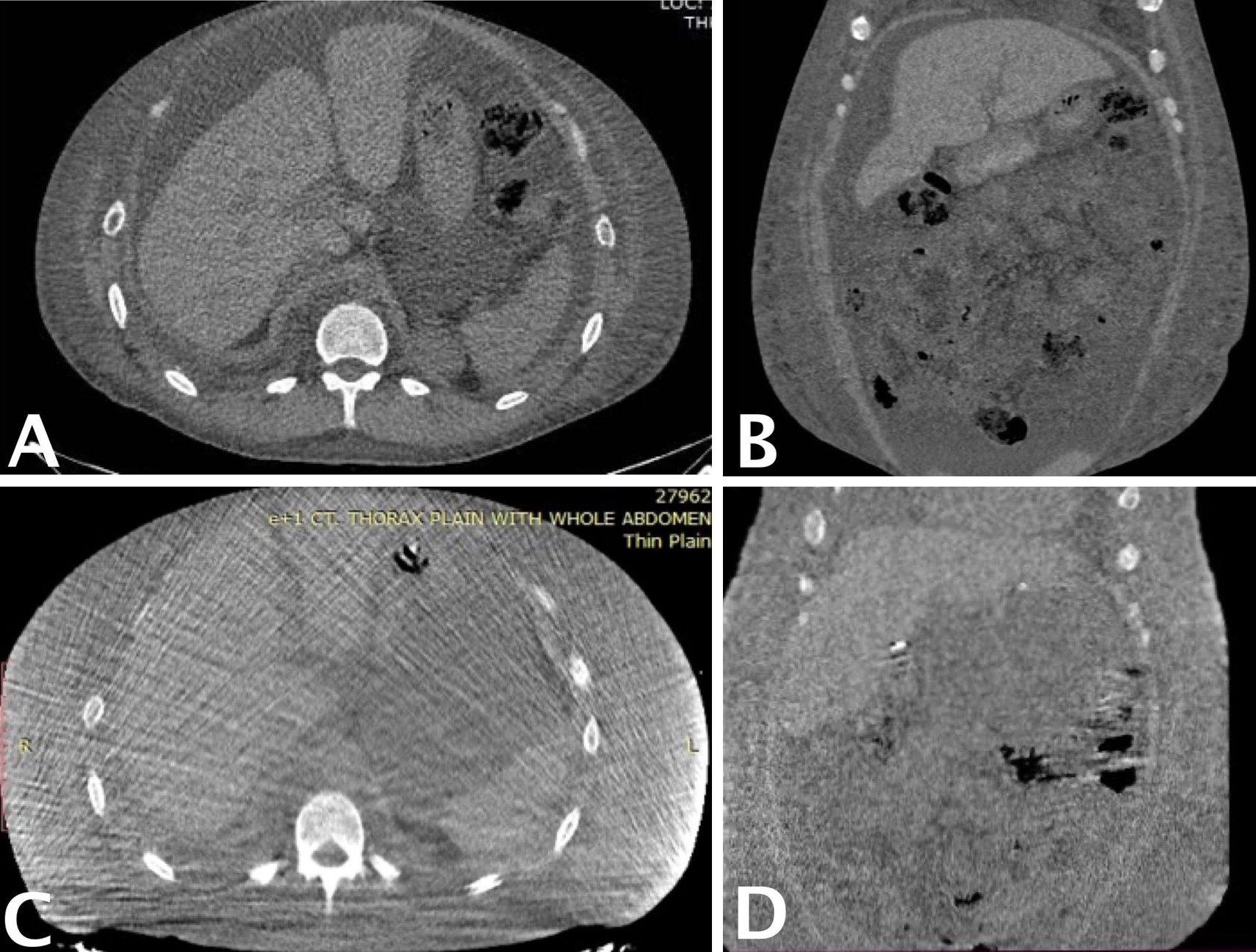
At the one-year follow-up, abdominal computed tomography (Figure 4) confirmed sustained resolution of chylous ascites, with no evidence of recurrence. However, mild residual pleural effusion and persistent lower limb edema were observed, findings suggestive of ongoing chronic hypoalbuminemia and active SLE. Serum albumin levels, initially 2 g/dL prior to surgery, transiently improved to 3 g/dL following perioperative supplementation but declined to 2 g/dL by the one-year evaluation.
Overall, the patient remained clinically stable, with no signs of flap ischemia, perfusion compromise, or abdominal wall herniation. Owing to geographic constraints and limited transportation access, follow-up beyond the one-year mark was conducted primarily through structured telephone consultations.
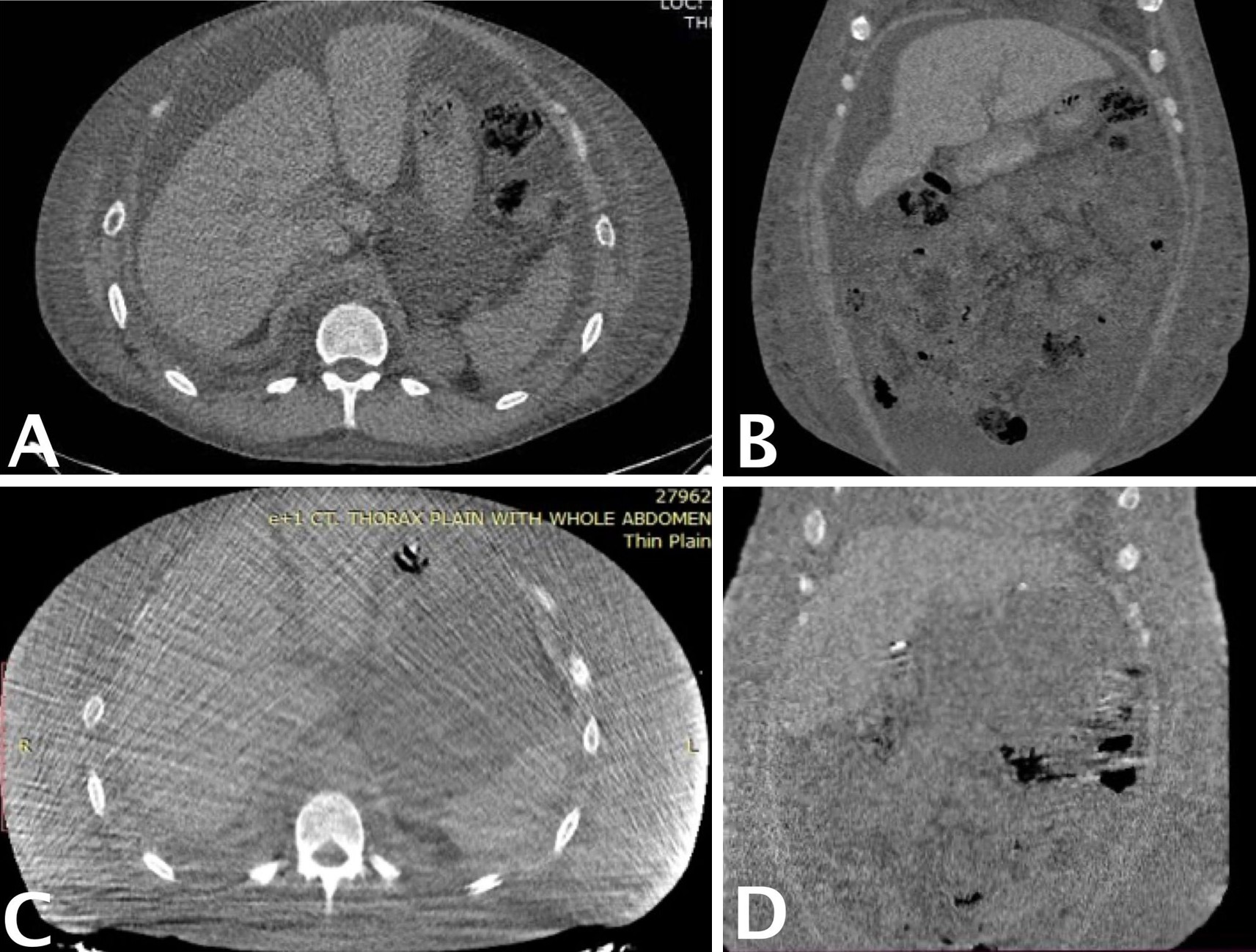
Figure 4. Preoperative and one-year postoperative abdominal CT demonstrating resolution of chylous ascites. (A) Preoperative axial CT image showing moderate to severe ascites, with large homogeneous low-density fluid collections surrounding intra-abdominal organs. (B) Preoperative coronal CT image further illustrating the bilateral distribution of ascitic fluid. (C) One-year postoperative axial CT image showing complete resolution of ascites and restoration of normal intra-abdominal anatomy. (D) One-year postoperative coronal CT image confirming absence of fluid accumulation and clear visualization of abdominal structures. CT, computed tomography.
Methods
To examine the clinical features, treatment modalities, and outcomes of chylous ascites associated with SLE, an integrative literature review was conducted. Available case reports and clinical studies were synthesized to construct a preliminary evidence-based framework to guide clinical decision-making.
Data were retrieved from multiple medical databases and open-access platforms. Eligible materials included peer-reviewed case reports, case series, and narrative studies. Citation tracing and conference abstracts were reviewed to enhance data completeness. The search strategy employed combinations of terms including "systemic lupus erythematosus," "chylous ascites," and "chylothorax," and encompassed all relevant publications available through March 2025. Non-English sources were included if clinically interpretable.
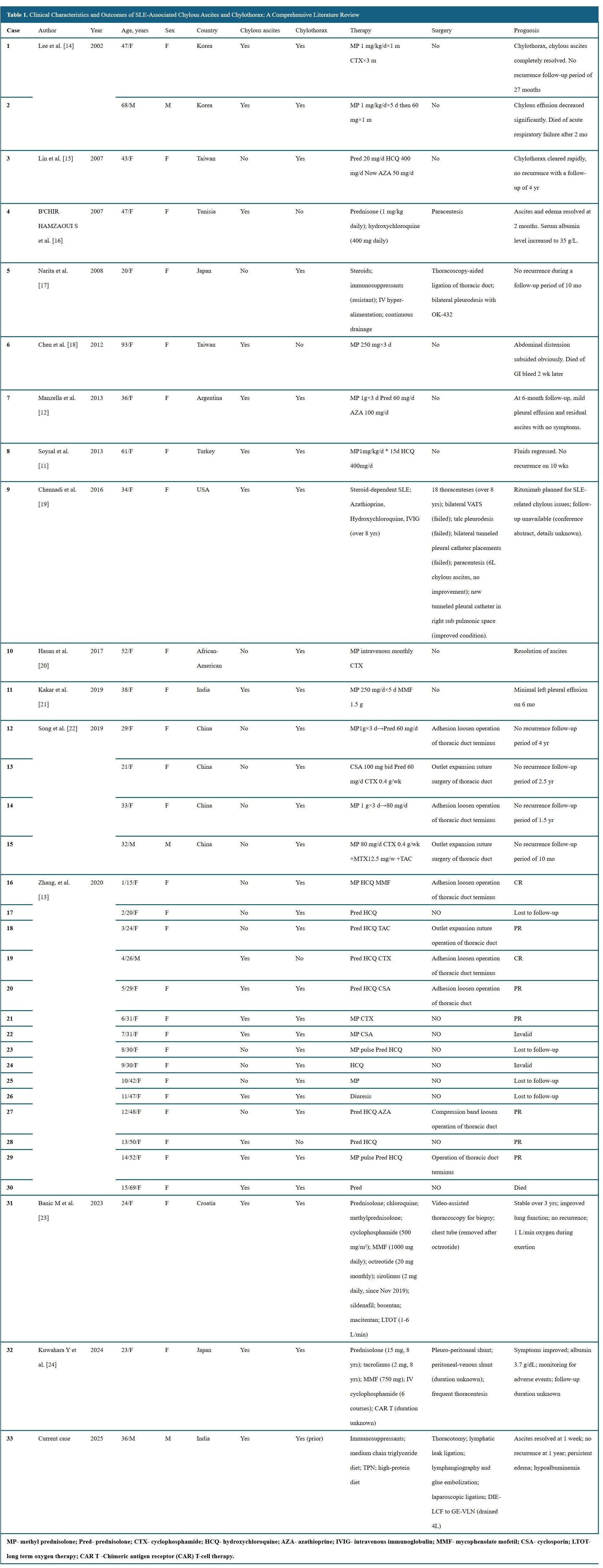
Inclusion criteria comprised: (1) a confirmed diagnosis of SLE; (2) documentation of chylous ascites with clinical or laboratory confirmation; and (3) reported data on presentation, treatment, or outcomes. Exclusion criteria included: (1) cases reporting chylothorax without ascites; (2) reports lacking essential information; and (3) chylous ascites unrelated to SLE. Eighteen published cases met the criteria and were included alongside the present case, resulting in 19 cases for analysis (Table 1) [5–14].
Data extraction focused on demographics (age, sex, and geographic distribution), clinical characteristics (including chylothorax), therapeutic interventions, and outcomes. Extracted data were verified against original texts to ensure consistency. Descriptive statistics and cross-sectional comparisons were used to identify trends in epidemiologic patterns and treatment responses.
Given the rarity of this condition and the predominance of single-case literature, a narrative review methodology was adopted. Although not a formal systematic review, this integrative analysis was conducted with methodological discipline, transparency, and internal consistency to ensure the reliability and clinical relevance of its findings. This approach aims to provide pragmatic insights into the management of SLE-associated chylous ascites and to inform future research on this rare but clinically significant complication.
Epidemiology
Among the 19 cases of SLE-associated chylous ascites included in this analysis, 15 cases (79%) were accompanied by concurrent chylothorax. This observation suggests that SLE-related lymphatic dysfunction frequently manifests as multicompartimental chyle leakage.
The majority of patients were female (84%, 16 of 19), aligning with the well-established female predominance in SLE. Patient age ranged from 23 to 93 years, with a median of 38 years, indicating that this complication can arise across a wide age spectrum.
The largest proportion of cases originated from China (8 of 19, 42%), followed by South Korea and India (2 of 19 each, 11%). Additional cases were reported from Tunisia, Taiwan, Argentina, Turkey, the United States, Croatia, and Japan, each contributing one case (5%).
Conservative Management
Immunosuppressive therapy remains the foundation of treatment for SLE-associated chylous ascites. Corticosteroids were employed as first-line agents in 18 of 19 cases (95%). Additional immunosuppressants were selected according to disease severity and organ involvement, including hydroxychloroquine (7 of 19, 37%), cyclophosphamide (5 of 19, 26%), and mycophenolate mofetil (3 of 19, 16%). In refractory cases, cyclosporine (2 of 19, 11%) and experimental modalities such as chimeric antigen receptor (CAR) T-cell therapy (1 of 19, 5%) were administered, reflecting a growing interest in targeted immunomodulation.
Supportive interventions, such as dietary modification with medium-chain triglyceride supplementation, were described in only one case (5%), suggesting that nutritional strategies remain underutilized in routine clinical practice.
Despite the central role of immunosuppression, outcomes with conservative therapy alone appear limited. Among the 11 patients managed exclusively with medical treatment, only 2 achieved complete remission, corresponding to a success rate of 18%. These findings underscore the potential inadequacy of immunosuppression alone in addressing the multifactorial pathophysiology of SLE-associated chylous ascites, particularly in the presence of persistent lymphatic obstruction and chronic inflammation.
For refractory cases, a multimodal approach may be warranted. Combining immunosuppressive therapy with nutritional optimization and lymphatic reconstructive procedures may improve therapeutic response and support sustained clinical remission.
Surgical Treatment
Of the 19 patients included in this review, 8 (42%) underwent surgical intervention in addition to immunosuppressive therapy. Among these, 7 received conventional procedures such as paracentesis, peritoneovenous shunting, or thoracic duct repair. However, only 3 of these 7 patients achieved complete remission, yielding a success rate of 43%. Although this represents a modest improvement over conservative therapy alone, the overall effectiveness of traditional surgical strategies remains limited. These findings underscore the difficulty of resolving lymphatic obstruction in SLE using localized or symptom-targeted interventions.
In contrast, the present case (Case 19 in Table 1) demonstrated complete resolution of chylous ascites within one week following DIE-GE lymphatic bypass. No recurrence or hernia-related complications were observed throughout the one-year follow-up period, and the patient's clinical condition remained stable. This successful outcome increased the complete remission rate for combined medical and surgical management from 43% to 50% (4 of 8 cases). This favorable course suggests that a systemically oriented lymphatic bypass may offer advantages over conventional focal interventions, particularly in cases involving diffuse or inflammatory lymphatic disruption associated with autoimmune disease.
The DIE-GE lymphatic bypass offers a novel surgical strategy for managing refractory chylous ascites in patients with SLE. By correcting both anatomical disruption and lymphatic dysfunction, it provides a promising foundation for advancing surgical care in autoimmune-related lymphatic disorders.
Current Case Analysis
Chylous fluid accumulation in the peritoneal, pleural, or pericardial cavities is often driven by elevated intraluminal pressure within central lymphatic conduits. This increase in pressure typically results from structural obstruction of major lymphatic channels, such as the lumbar trunks, cisterna chyli, and thoracic duct. These features are characteristic of central conducting lymphatic anomaly, a condition marked by impaired lymphatic transport.
In rare instances, SLE may contribute to such obstructions through chronic autoimmune-mediated inflammation. This inflammatory process can lead to progressive narrowing or dysfunction of central lymphatic pathways. As a result, pathological chyle leakage may occur, contributing to the complex lymphatic manifestations observed in select patients with SLE [8,9].
Direct lymphangiography is a valuable diagnostic modality for assessing thoracic duct patency and identifying outflow obstruction, thereby informing the feasibility of surgical decompression and symptom management strategies [12]. In the present case, however, lymphangiography did not demonstrate opacification of the thoracic duct, and no definitive anatomical obstruction was observed on prior imaging. These findings raise the possibility that the chylous ascites may be attributable to diffuse microlymphatic dysfunction, rather than to a discrete anatomical blockage.
Challenges in conservative management
Although the treatment of chylous ascites is well established [1,15], cases associated with SLE remain rare. This limited clinical experience hampers diagnostic precision and constrains the development of standardized therapeutic strategies. The present case illustrates the persistent challenges encountered during management of this uncommon condition.
The patient exhibited minimal response to multiple conservative interventions, including immunosuppressive therapy, dietary modification, and serial paracenteses, none of which achieved durable clinical benefit. A subsequent attempt at laparoscopic ligation of the suspected chyle leak was also unsuccessful [16]. While the Denver shunt has been reported as a palliative option, its limited long-term efficacy and risks such as infection and thrombosis diminished its clinical appeal [17]. The patient therefore declined this intervention.
Thoracic duct embolization has been proposed as a targeted treatment for proximal chyle leakage [18]. However, in this case, the procedure could not be performed. During direct lymphangiography, contrast failed to ascend from the inguinal lymph nodes, precluding thoracic duct opacification and obstructing both diagnostic and therapeutic access.
This scenario underscores the limitations of conventional approaches, particularly when central lymphatic anatomy cannot be adequately visualized or when diffuse microlymphatic dysfunction is present. In cases associated with SLE, such challenges are further complicated by chronic autoimmune inflammation. These observations highlight the pressing need for innovative, mechanism-based interventions capable of addressing complex lymphatic dysfunction in refractory disease.
Redefining surgical strategy
Given the limitations of conventional treatment, the surgical management of SLE-associated chylous ascites warrants fundamental reassessment. Strategies must be adapted to the condition’s multifactorial pathophysiology and immunological complexity. For cases involving distal thoracic duct obstruction, thoracic duct to venous bypass has been proposed as a viable option [19]. In the present patient, however, the presumed disruption was proximal, beyond the anatomical reach of this technique.
When chyle leakage is anatomically localized, intra-abdominal lymphovenous anastomosis may serve as an alternative [20]. In this case, however, repeated paracenteses resulted in bacterial peritonitis, rendering microsurgical peritoneovenous bypass contraindicated due to the heightened infection risk [21].
These limitations highlight the need for innovative surgical solutions that balance anatomical versatility with immunological safety. Techniques that restore lymphatic flow without requiring precise leak localization may offer greater therapeutic efficacy. The DIE-GE lymphatic bypass performed in this case exemplifies such an approach and points toward a promising surgical direction for refractory chylous ascites in SLE.
Conceptual basis for innovation
In response to the multifaceted therapeutic limitations in managing SLE-associated chylous ascites, this study explored a novel surgical strategy centered on microsurgical reconstruction of lymphatic outflow. Acknowledging the limited efficacy of both conservative medical approaches and anatomically localized surgical interventions, we adapted the principles of vascularized lymph node transfer (VLNT) to address diffuse lymphatic dysfunction within an inflamed immunological environment.
VLNT is a well-established treatment for limb lymphedema, where transplanted lymph nodes facilitate both lymphatic drainage and local immunoregulation [22]. Extending this paradigm, we employed the DIE-GE lymphatic bypass as a modified VLNT technique tailored to the autoimmune context of SLE. Drawing from the original approach described by Ciudad et al. [4], the procedure was structurally refined to optimize anatomic integration, reduce perioperative risks, and enhance functional outcomes in the setting of chronic inflammatory lymphatic compromise.
Optimizing flap inset
The DIE-GE lymphatic bypass was initially developed for non-SLE cases of lymphatic obstruction. By diverting chyle into the extraperitoneal circulation, the technique bypasses intra-abdominal lymphatic blockages and facilitates sustained drainage [4]. Building on this foundation, we introduced technical refinements to address pathological features common in SLE, such as chronic inflammation, tissue fragility, and impaired wound healing. In this case, morbid obesity (body mass index >35 kg/m²) further increased the risk of postoperative herniation, necessitating a modified approach to flap inset and fixation that prioritized anatomical stability and long-term structural integrity.
To mitigate these risks, a cruciate incision was fashioned in the posterior rectus sheath at the junction of the upper and middle thirds of the rectus abdominis. The opening was precisely tailored to allow atraumatic passage of the GE-VLN pedicle while minimizing dead space that could predispose to displacement or instability. The retrorectus plane was dissected to a sufficient lateral extent to accommodate the flap without inducing compressive stress.
To prevent prolapse or torsion, the flap construct was securely anchored to both the parietal peritoneum and the rectus sheath. The posterior rectus sheath was loosely reapproximated several centimeters above and below the cruciate incision to minimize tension at the suture line. The anterior rectus sheath was then closed in layered fashion using standard techniques, reinforcing abdominal wall integrity and reducing the risk of postoperative ventral hernia.
In standard surgical practice, intestinal stomas are routinely passed through the rectus musculature without compromising vascular integrity, supporting the anatomical feasibility of this region. Extrapolating from this principle, the retrorectus plane was considered unlikely to impose significant compressive risk on the flap. However, in patients with SLE, poor tissue resilience markedly increases the risk of incisional herniation if fascial closure is incomplete. These considerations underscored the necessity of adopting a modified inset technique.
The refined approach appeared effective, resulting in sustained resolution of ascites without radiologic evidence of herniation. No abdominal bulging or discomfort was reported during follow-up, supporting the structural soundness of the modified flap inset strategy.
Proposed drainage mechanisms
Findings from this case, together with prior reports, indicate that the DIE-GE lymphatic bypass ameliorates SLE-associated chylous ascites through multiple complementary mechanisms. These synergistic effects may account for the clinical improvements observed in the setting of autoimmune-mediated lymphatic dysfunction. Specifically, the therapeutic benefit appears to arise from three principal mechanisms: mechanical restoration of lymphatic drainage, local immunomodulation, and the promotion of lymphangiogenesis. Each is elaborated below.
The primary mechanism involves the reestablishment of an alternative lymphatic drainage pathway. Chylous fluid within the peritoneal cavity is redirected into the lymphoid tissue of the GE-VLN flap and subsequently drained into the deep inferior epigastric and gastroepiploic venous systems via microvascular anastomoses. The relatively high-pressure arterial inflow from the gastroepiploic artery is hypothesized to augment lymphatic flow within the DIE-LCF, thereby facilitating the bypass of obstructed intra-abdominal lymphatics and enhancing systemic chyle clearance [4,23].
The second mechanism pertains to immunomodulation. The vascularized lymphoid tissue within the GE-VLN flap has been shown to promote anti-inflammatory cytokine expression, particularly interleukin 10, while concurrently suppressing proinflammatory mediators such as tumor necrosis factor alpha [24]. This localized immunoregulatory effect is especially pertinent in SLE, a condition characterized by chronic systemic inflammation and immune dysregulation. In such settings, the long-term patency and functional stability of the drainage pathway may depend on sustained immunomodulatory support. Unlike previous applications in patients with lymphatic disruption from renal transplantation or malignancy-related interventions [4], individuals with SLE may derive unique benefit from the flap’s intrinsic capacity to modulate immune responses, thereby offering additional therapeutic advantage.
The third mechanism involves lymphangiogenesis. The transplanted lymphatic-rich tissue within the GE-VLN flap may stimulate the formation of new lymphatic channels, potentially through the upregulation of lymphangiogenic mediators such as vascular endothelial growth factor C [4,23]. This regenerative process could enhance microlymphatic circulation and reinforce the long-term integrity of lymphovenous connections, thereby improving fluid clearance and lowering the risk of recurrence.
The DIE-GE lymphatic bypass appears to exert its therapeutic effects through multiple synergistic mechanisms. In this case, ascites resolved completely within one week postoperatively. Follow-up imaging confirmed the absence of herniation or flap ischemia, reinforcing the feasibility of this approach for autoimmune-mediated lymphatic disorders.
DIE-GE Lymphatic Bypass: SLE vs. Non-SLE
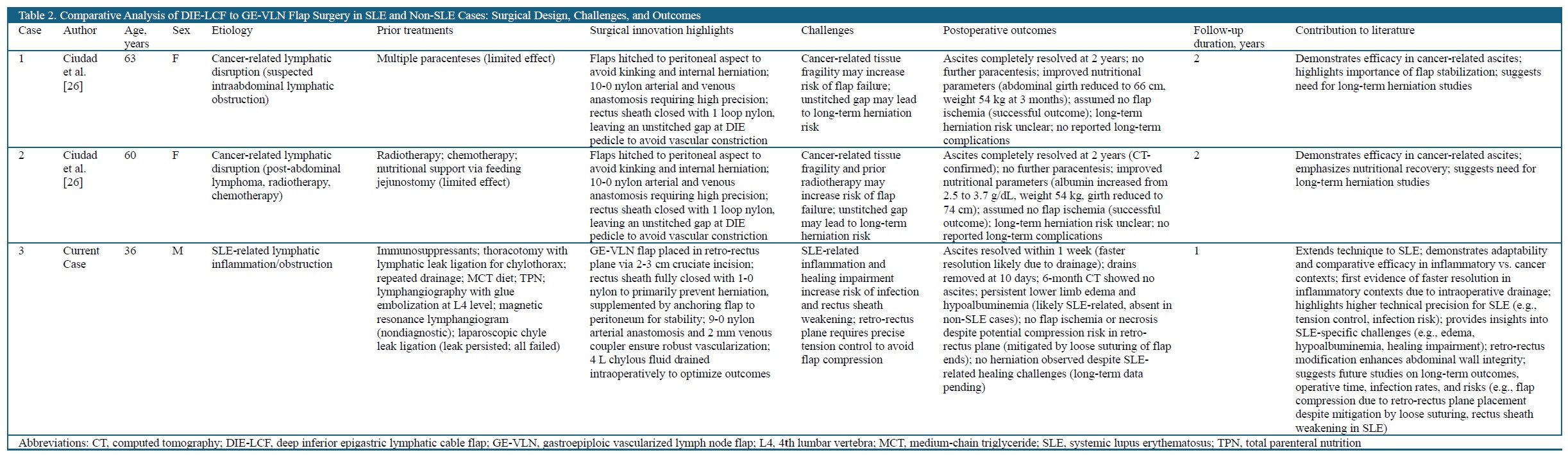
Since its introduction by Ciudad et al. [4], the DIE-GE lymphatic bypass has been applied only in two non-SLE cases involving mechanical lymphatic obstruction after renal transplantation or cancer therapy. In contrast, SLE-associated chylous ascites presents added complexity due to chronic inflammation, immune dysregulation, and poor tissue healing. This case represents the first successful adaptation of the procedure in an autoimmune setting, extending its potential use beyond purely mechanical causes. A comparative analysis of SLE and non-SLE cases, summarized in Table 2, highlights key disease-specific challenges and technical refinements, offering guidance for broader clinical application in complex lymphatic disorders.
Customized technique design
In non-SLE cases (Cases 2 and 3 in Table 2), the DIE-GE lymphatic bypass was performed for chylous ascites secondary to surgical or therapy-induced lymphatic obstruction, with surgical priorities focused on flap perfusion and abdominal wall preservation. Microvascular anastomoses were completed using 10-0 nylon, and a small gap was left in the anterior sheath to prevent pedicle compression, with no herniation observed over two years. In contrast, the SLE case required tailored modifications to address systemic inflammation and poor healing. The GE-VLN flap was inset into the retrorectus plane via a cruciate incision, secured with full fascial closure and peritoneal anchoring to ensure structural stability. These adjustments underscore the importance of etiology-specific strategies in optimizing surgical outcomes.
Pathophysiological challenges
Postoperative management in lymphatic surgery is shaped not only by etiology but also by systemic factors such as immune status, tissue resilience, and healing capacity. In non-SLE cases, strategies like leaving a small fascial gap reduce pedicle compression but may increase hernia risk, though no such complications have been reported to date. SLE poses greater challenges due to chronic inflammation, immune dysregulation, and poor wound healing, necessitating reinforced fixation and tension control during flap inset. In this case, persistent hypoalbuminemia and edema further prolonged recovery. These factors highlight the need for an integrated approach combining immune modulation, nutritional support, and structural optimization to improve outcomes in autoimmune lymphatic reconstruction.
Postoperative outcomes
The DIE-GE lymphatic bypass achieved effective local control of chylous ascites in both SLE and non-SLE patients. However, postoperative courses differed markedly. In non-SLE cases, gradual resolution occurred over two years without recurrence or complications. Nutritional status also improved steadily during follow-up. In contrast, the SLE case showed rapid resolution within one week, plausibly aided by intraoperative chyle evacuation. Nonetheless, recovery was complicated by persistent lower limb edema and hypoalbuminemia, findings that reflect ongoing systemic disease activity. Despite theoretical concerns regarding flap compression within the confined retrorectus space, no ischemia or herniation was observed. These outcomes support the safety of complete fascial closure. They also underscore the additional complexity of postoperative care in autoimmune disease. A multidisciplinary approach integrating immunologic, nutritional, and structural support is essential to ensure sustained clinical benefit.
Cross-etiology implications
The postoperative outcomes in this study demonstrate the adaptability of the DIE-GE lymphatic bypass across diverse etiologies. In non-SLE cases, the technique effectively managed lymphatic obstruction following transplantation or malignancy, achieving durable ascites control and improved nutritional recovery. However, intentional nonclosure of the anterior rectus sheath to protect the DIE pedicle may pose a latent herniation risk, warranting long-term surveillance.
By contrast, this is the first report of DIE-GE application in SLE-associated chylous ascites, a condition driven by immune-mediated lymphatic dysfunction and impaired tissue repair. The successful outcome highlights the feasibility of this approach in autoimmune contexts when combined with tailored refinements, such as retrorectus flap placement and complete fascial closure. These adaptations address tissue fragility while maintaining structural stability, offering a potential surgical model for lymphatic reconstruction in inflammatory diseases.
Challenges in SLE Chylous Ascites
To better define the unique challenges of SLE-associated chylous ascites, we conducted a systematic analysis incorporating the present case and 18 published reports (Table 1). This evaluation examines epidemiological trends, treatment responses, and existing gaps, aiming to clarify disease characteristics and inform future diagnostic and therapeutic strategies.
Demographic characteristics
Among the 19 reported cases of SLE-associated chylous ascites, 84% (16 of 19) occurred in female patients, with a median age of 38 years (range, 23–93), consistent with the established epidemiological profile of SLE [9]. Geographically, cases were documented across Asia, Africa, Europe, and the Americas, confirming a global presence despite limited recognition. A disproportionate number originated from China (8 of 19, 42%), compared with South Korea and India (2 each), and single reports from Tunisia, Taiwan, Argentina, Turkey, the United States, Croatia, and Japan. This skewed distribution may reflect regional reporting bias, heightened clinical awareness, or a greater tendency to publish rare SLE manifestations.
Notably, 79% (15 of 19) of patients also developed chylothorax, indicating that SLE-related chylous effusions often involve multiple anatomical compartments. This pattern is compatible with systemic lymphatic disruption driven by chronic inflammation or immune-mediated injury to central conduits such as the thoracic duct and cisterna chyli. Awareness of this multichamber tendency is clinically important when evaluating unexplained effusions in patients with active SLE [1,8].
Therapeutic limitations
Therapeutic outcomes in SLE-associated chylous ascites remain limited. Among 19 reported cases, complete remission was achieved in only 18% of patients treated with conservative therapy alone, rising to 43% when combined with conventional surgery. Despite this modest improvement, overall efficacy remained suboptimal. The mortality rate was 16% (3 of 19), with all deaths occurring in patients aged 68 to 93 years. This association suggests that advanced age confers increased vulnerability, likely due to coexisting immune dysfunction, comorbid conditions, and diminished physiologic reserve. These findings highlight the limitations of monotherapy and support early risk stratification and tailored multimodal strategies integrating immunosuppression, nutritional optimization, and reconstructive surgery.
Toward a new therapeutic direction
Conventional therapies for SLE-associated chylous ascites remain inadequate, primarily due to the diffuse lymphatic disruption and chronic inflammation characteristic of the disease. Localized interventions often provide only temporary relief without sustained control. The current case (Case 19 in Table 1) introduces the DIE-GE lymphatic bypass as an innovative approach that combines microsurgical vascular anastomosis with vascularized lymph node transfer to redirect chyle into the systemic circulation. In contrast to conventional surgical procedures, the present case demonstrated complete and sustained remission following DIE-GE lymphatic bypass.
This outcome raised the overall complete remission rate among surgically managed patients to 50% (4 of 8), compared to 43% for conventional procedures alone. The favorable response observed suggests that DIE-GE lymphatic bypass may represent a promising microsurgical option when both standard immunosuppression and traditional surgical approaches prove inadequate. In addition to mechanical diversion, this technique may offer immunological and regenerative benefits through local immunomodulation and lymphangiogenesis. Although based on a single patient, the rapid resolution of chylous ascites and sustained clinical stability observed support the therapeutic potential of this intervention. Broader validation and standardized protocols, along with integrated postoperative care, are essential to advance treatment in this complex setting.
Study Limitations
Several limitations should be acknowledged. SLE-associated chylous ascites is exceedingly rare, and current literature consists primarily of isolated case reports, which may introduce selection and publication bias and limit generalizability. In this study, follow-up beyond one year was conducted through structured telephone consultations due to geographic constraints, which may have limited the detection of delayed complications such as herniation, perfusion deficits, or fibrosis. The proposed mechanisms of therapeutic benefit, including lymphatic rerouting, immunologic modulation, and lymphangiogenesis, were not directly verified and require further confirmation through dedicated imaging or histologic analysis. Although the successful application of rectus sheath closure and flap fixation demonstrates technical feasibility in this case, broader validation across diverse SLE subtypes and disease severities is essential to determine scalability and refine surgical selection criteria.
This study presents the first comprehensive review of SLE-associated chylous ascites, offering an evidence-based framework for therapeutic comparison and addressing a critical gap in clinical understanding. Both pharmacologic and conventional surgical approaches demonstrated limited efficacy, particularly in refractory cases. Within this context, the current case represents the first successful application of the DIE-GE lymphatic bypass in SLE, achieving complete ascites resolution and sustained postoperative stability. These findings suggest that the technique may offer a durable therapeutic option for autoimmune lymphatic disorders. Further studies are warranted to confirm its safety, optimize surgical protocols, and evaluate broader clinical utility.
Received date: March 10, 2025
Accepted date: May 19, 2025
Published date: June 11, 2025
We gratefully acknowledge the contributions of the multidisciplinary medical team at our institution for their expertise and dedicated care in the evaluation and management of this patient.
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2025 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
A significant increase in peripheral nerve surgery has occurred in recent years due to improvements in surgical techniques. In most reconstructive procedures, sensory restoration is frequently neglected in preference to restoring motor function. Along with increasing the risk of developing injuries to the body, patients who lose protective sensations are more likely to develop neuropathic pain and depression, which adversely affect their quality of life. As regaining sensory function is important, the study examines a variety of techniques that may be useful for restoring sensory function across various body parts.
This study introduces an advanced tubularized radial artery forearm flap (RAFF) technique, marking an enhancement over traditional methods in addressing complex nasal reconstructions. It integrates functional and aesthetic considerations through a structured, multi-stage reconstruction process, emphasizing the use of tubularized flaps. Key learning points include the detailed crafting of stable nasal passages, strategic use of costal cartilage for robust structural support, and tailored postoperative care with silicone splints. The tubularized RAFF technique not only optimizes patient outcomes and quality of life but also provides plastic surgeons with critical insights to refine their techniques in facial reconstruction. Indispensable for professionals in the field, this article enriches the understanding of sophisticated reconstructive challenges and solutions.
This article presents a crucial case report on potential wound healing complications linked to fremanezumab, a calcitonin gene-related peptide-targeting antibody for migraine prevention. It documents the first known instance of delayed wound healing following a free flap breast reconstruction, underscoring the need for heightened clinical vigilance and individualized patient assessment in perioperative settings. Highlighting significant safety data gaps, the report advocates for comprehensive research and rigorous post-marketing surveillance. The findings emphasize the importance of balancing the risks of delayed wound healing with the need for effective disease control, especially when using biologic agents for chronic conditions. This article is essential for medical professionals managing patients on biologic therapies, offering critical insights and advocating for a personalized approach to optimize patient outcomes. By presenting novel observations and calling for further investigation, it serves as a vital resource for enhancing patient care and safety standards in the context of biologic treatments and surgical interventions.
This manuscript showcases an advanced surgical approach for treating malignant giant cell tumor of bone, emphasizing precision and ethical considerations. It leverages innovative pedicled flap technologies, as opposed to free flaps, enhancing limb functionality and patient quality of life. This technique equips surgeons with evidence that tailored surgical strategies can significantly improve outcomes in complex cases. The paper discusses technical challenges and highlights the application of supercharging and superdrainage techniques in limb reconstructions, methods well-established in microsurgery but infrequently used in oncological contexts. These techniques are crucial for optimizing flap viability and ensuring surgical success. Additionally, the manuscript underscores the profound impact of these advancements on patient lives, offering hope and showcasing tangible benefits. This narrative, blending scientific analysis with patient stories, enriches the understanding of limb reconstruction innovations in oncological surgery, making it invaluable for surgeons.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
All three reviewers commended the study for its innovative application of the DIE-LCF to GE-VLN flap anastomosis technique in the management of refractory chylous ascites in a patient with SLE, emphasizing its significant clinical potential. However, the reviewers consistently note that the mechanisms underlying the procedure (e.g., the connection to CCLA and the resolution process) and technical details (e.g., incision design and vascular pedicle management) could benefit from further clarification and analysis. Reviewer 1 raises concerns about the potential risk of flap compression, Reviewer 2 points out areas where the rationale for VLNT could be strengthened, and Reviewer 3 suggests including additional details on the patient’s SLE history and albumin data to enhance the study’s comprehensiveness.
In my view, the authors have not yet fully showcased the distinctive value of their work. First, SLE-related chylous ascites is exceptionally rare, with only 32 cases documented in the literature, most of which rely on non-surgical treatments with limited success. This study is the first to apply DIE-LCF to GE-VLN anastomosis for SLE-associated chylous ascites, offering a promising new approach for refractory cases. Second, while this technique was previously used only for cancer-related chylous ascites, this study successfully extends its application to SLE, opening a new clinical avenue and providing a potential breakthrough for managing lymphatic complications in SLE, which are associated with high mortality rates.
To further elevate the study’s academic impact, I recommend incorporating two comparative tables. Table 1 could summarize the 33 reported cases of SLE-related chylous ascites or chylothorax (including the current case), comparing clinical characteristics and outcomes. Table 2 could contrast the current case with the two prior DIE-LCF to GE-VLN cases to highlight key differences. Our editorial team is pleased to provide table templates (Suggested Tables 1 and 2 for inclusion) to help highlight the unique contribution of this case to the management of SLE-associated chylous ascites. With these revisions, the manuscript is well-positioned to serve as a pivotal reference in SLE lymphatic treatment and has strong potential for publication.
ResponseWe thank the editor for the valuable additions to the article. These have been incorporated in the manuscript (Table 1, line 118, page 6; Table 2, line 149, page 7). As a methodological note, the scope of the present study is limited to cases of SLE-associated chylous ascites. Therefore, cases involving chylothorax without accompanying ascites (n = 14) were excluded from the comparative analysis to ensure clinical consistency and analytic rigor.
This study presents an innovative microsurgical technique for treating refractory chylous ascites in a 36-year-old patient with SLE. The technique uses a DIE-LCF anastomosed to a GE-VLN. The flaps were placed in the retro-rectus plane to reduce herniation risk. Post-surgery, ascites resolved within one week. A 6-month CT scan confirmed sustained resolution. These outcomes highlight significant clinical potential. Building on the technique reported by Ciudad et al., this study enhances structural stability by placing the GE-VLN flap in the retro-rectus plane and fully closing the rectus sheath. However, the potential for flap compression in the retro-rectus plane and the long-term effects of the cruciate incision warrant further exploration. Additional discussion of technical details, such as incision design and vascular pedicle management, would enrich the analysis. I suggest the authors elaborate on these technical details to enhance the manuscript’s readability, scientific rigor, and impact.
A 36-year-old patient with SLE presented with persistent, refractory chylous ascites. Conventional treatments failed to provide relief. The authors introduced a novel microsurgical technique, anastomosing a DIE-LCF to a GE-VLN, offering a new therapeutic pathway for complex lymphatic disorders. The case underscores the clinical value of this approach. However, the discussion of the mechanism underlying ascites resolution lacks clarity, supporting literature, and specific hypotheses. Additionally, the mention of VLNT is abrupt and its relevance to chylous ascites is insufficiently explained. Revising the discussion to propose plausible mechanisms, supported by relevant literature, and clarifying the role of VLNT would enhance the logical flow and academic depth of the manuscript.
This is a case report of a 36-year-old male patient with SLE who experienced refractory chylous ascites. Treatments such as immunosuppressants, thoracic duct ligation, laparoscopic surgery, and glue embolization failed. He was referred to the Plastic Surgery department for a solution. The study presents an innovative microsurgical approach. It employs microvascular anastomosis of DIE-LCF to GE-VLN to bypass intra-abdominal lymphatic blockages. This drains chylous fluid into the systemic circulation. During surgery, 4 liters of chylous fluid were removed. One week after surgery, the ascites resolved. A 6-month CT scan confirmed no recurrence. Albumin levels improved. Unlike Ciudad et al.’s technique for non-autoimmune chylous ascites, this approach targets SLE’s inflammatory lymphatic obstruction. It addresses a gap in autoimmune treatment and redefines lymphatic disorder management. However, the connection to CCLA remains underexplored. Clinical data, such as albumin changes and drainage volume timelines, are limited. Adding numbers, like albumin levels before and after surgery, would make the report more scientifically solid.
Naalla R, Samantaray SA, Patil S, Rao GV, Reddy DN. Microsurgical lymphatic reconstruction for refractory chylous ascites in systemic lupus erythematosus: Case report and literature review. Int Microsurg J 2025;9(1):2. https://doi.org/10.24983/scitemed.imj.2025.00197