This case report explores the potential effects of fremanezumab, a calcitonin gene-related peptide-targeting antibody used for migraine prevention, on postoperative wound healing. To our knowledge, this is the first documented case of a female experiencing delayed wound healing after receiving fremanezumab following a free flap breast reconstruction. The patient, who tested positive for the BRCA gene mutation, underwent a bilateral prophylactic nipple-sparing mastectomy with a transversus rectus abdominis muscle flap reconstruction. Initially, recovery was uncomplicated, but severe skin necrosis and blistering were noted at the first postoperative visit. The condition worsened, requiring topical treatments and sharp debridement. Despite low-grade fevers and prophylactic antibiotic treatment, no infection was formally confirmed. Frequent debridement was necessary for several months. By five months postoperatively, the breasts and most abdominal wounds had healed. This case underscores the need for heightened clinical awareness, suggesting a potential association between fremanezumab and impaired wound healing. This observation has significant implications for perioperative patient management. Notably, while there is a potential link between fremanezumab and the impaired wound healing observed in this case, a direct causal relationship remains unconfirmed. It is crucial to carefully balance the risks of delayed wound healing with the potential for worsened disease control. This consideration is especially important when using biologic agents for chronic conditions. Each case should be evaluated individually to tailor the best treatment approach.
Calcitonin gene-related peptide (CGRP) is a vital vasoactive component of the trigeminovascular system. It plays a crucial role in the pathogenesis of migraine attacks when present in the bloodstream [1]. The development of CGRP monoclonal antibodies has pioneered a novel class of prophylactic treatments for chronic migraine [2]. Beyond its neurological impact, CGRP is also critical for wound healing. It promotes revascularization by upregulating vascular endothelial growth factor, decreases levels of inflammatory mediators such as tumor necrosis factor-alpha and macrophages, and stimulates proliferation of keratinocytes [3]. Consequently, deficiencies in CGRP can significantly impair wound healing processes.
The association between impaired wound healing and CGRP monoclonal antibodies is underscored by a case involving a 51-year-old migraine patient treated with erenumab [3]. Following minor injuries, this patient experienced severe wound healing complications, with biopsy results revealing extensive skin inflammation and vessel thrombosis. While this case highlights the potential side effects associated with erenumab, the impact of fremanezumab on wound healing remains less defined.
The clinical trials evaluating fremanezumab, detailed in its package insert, consisted of two multicenter, randomized, three-month, double-blind, placebo-controlled studies [4]. These studies, however, excluded patients with major cardiovascular or thrombotic conditions such as cerebrovascular accidents, transient ischemic attacks, deep vein thrombosis, or pulmonary embolisms. This exclusion highlights significant safety data gaps for patients with vascular disorders and those undergoing surgeries with major vascular implications. This underscores the imperative for targeted, comprehensive research.
We present a case of significantly impaired wound healing in a patient with chronic migraines treated with fremanezumab. This occurred following autologous free flap breast reconstruction after a bilateral mastectomy. To our knowledge, this is the first reported instance of wound healing delays linked to fremanezumab in breast reconstruction. The case underscores the urgent need for heightened clinical vigilance and suggests a potential connection between fremanezumab and delayed wound healing. It also calls for further research into its perioperative impacts.
A 48-year-old woman with a history of chronic migraines has been under management with fremanezumab since May 2021 for her condition. Following a positive test for the BRCA gene mutation, she consulted a plastic surgeon regarding preventive breast surgery. She subsequently underwent bilateral prophylactic nipple-sparing mastectomy with muscle-sparing transversus rectus abdominis muscle flap reconstruction. Notably, she did not present with conventional risk factors for poor wound healing such as obesity, smoking, or corticosteroid use.
Her regimen of monthly fremanezumab injections for chronic migraines continued uninterrupted in preparation for the elective surgery, spanning approximately 15 months prior to the procedure. Initially, her postoperative period was uneventful, and she was discharged on the third day without any immediate concerns for wound healing. However, early bruising patterns on her skin soon indicated potential complications, suggesting the possibility of future skin necrosis (Figure 1A). This led the surgical team to arrange close follow-up.
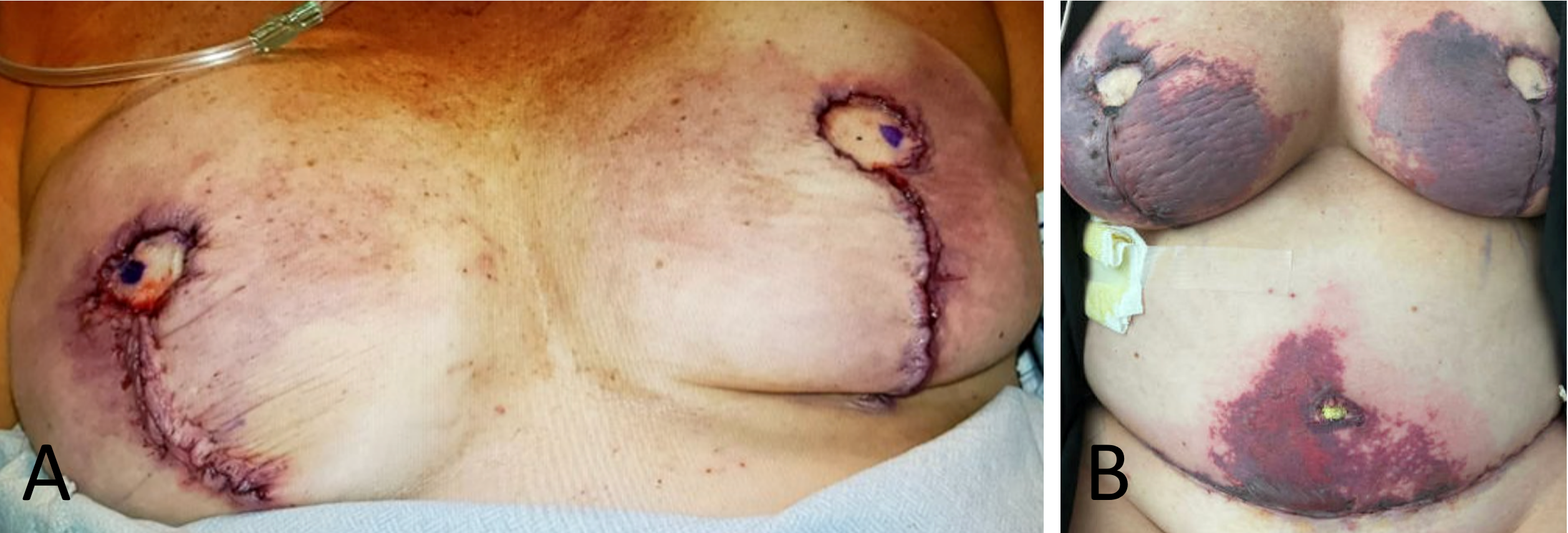
Figure 1. Initial and one-week postoperative observations following bilateral prophylactic mastectomy and reconstruction. (A) Immediately after surgery, this panel shows the surgical results of a bilateral prophylactic nipple-sparing mastectomy complemented by transversus rectus abdominis muscle flap reconstruction. The incisions are notably clean, exhibiting no immediate signs of complications. Nonetheless, early bruising patterns on the skin hint at potential complications and the risk of subsequent skin necrosis. (B) At one week postoperative, the image reveals pronounced skin necrosis and blistering at both the bilateral breast incisions and the abdominal donor site, underscoring a significant impairment in wound healing.
At her first postoperative examination one week later, significant skin necrosis and severe blistering at the incision sites were observed (Figure 1B). Subsequently, the wounds deteriorated further, exhibiting increased necrosis, purple discoloration, blistering, and sloughing (Figure 2). Consequently, treatment strategies were adjusted to include applications of Silvadene cream, Medihoney, and Hydrogel.
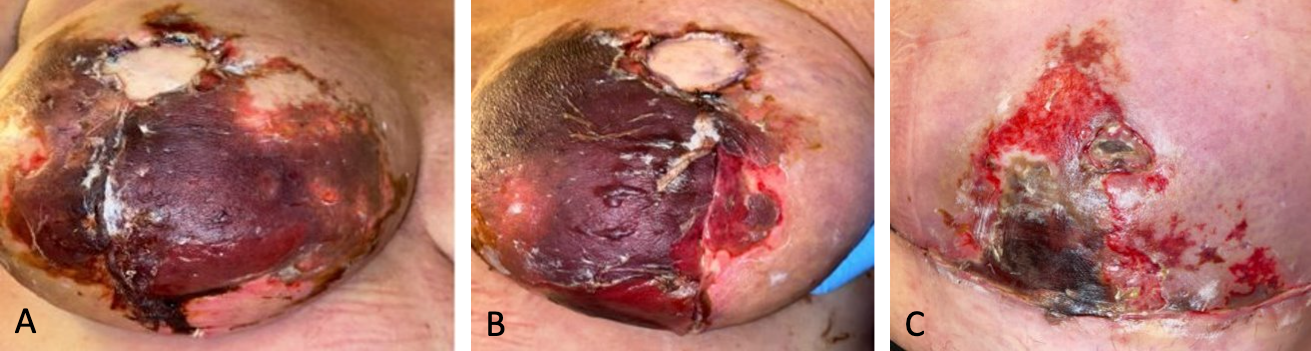
Figure 2. Evolving skin necrosis of surgical incisions. (A) Right breast showing increased necrosis and purple discoloration. (B) Left breast displaying extensive blistering and sloughing. (C) Abdominal donor site with significant necrosis and sloughing.
One month post-surgery, she developed low-grade fevers, prompting empirical treatment with doxycycline. As the fevers persisted, levofloxacin was administered to address potential Pseudomonas infections, despite the absence of confirmed cellulitis or surgical site infection. The extensive eschars over her bilateral breast and abdominal wounds eventually necessitated sharp debridement (Figure 3).
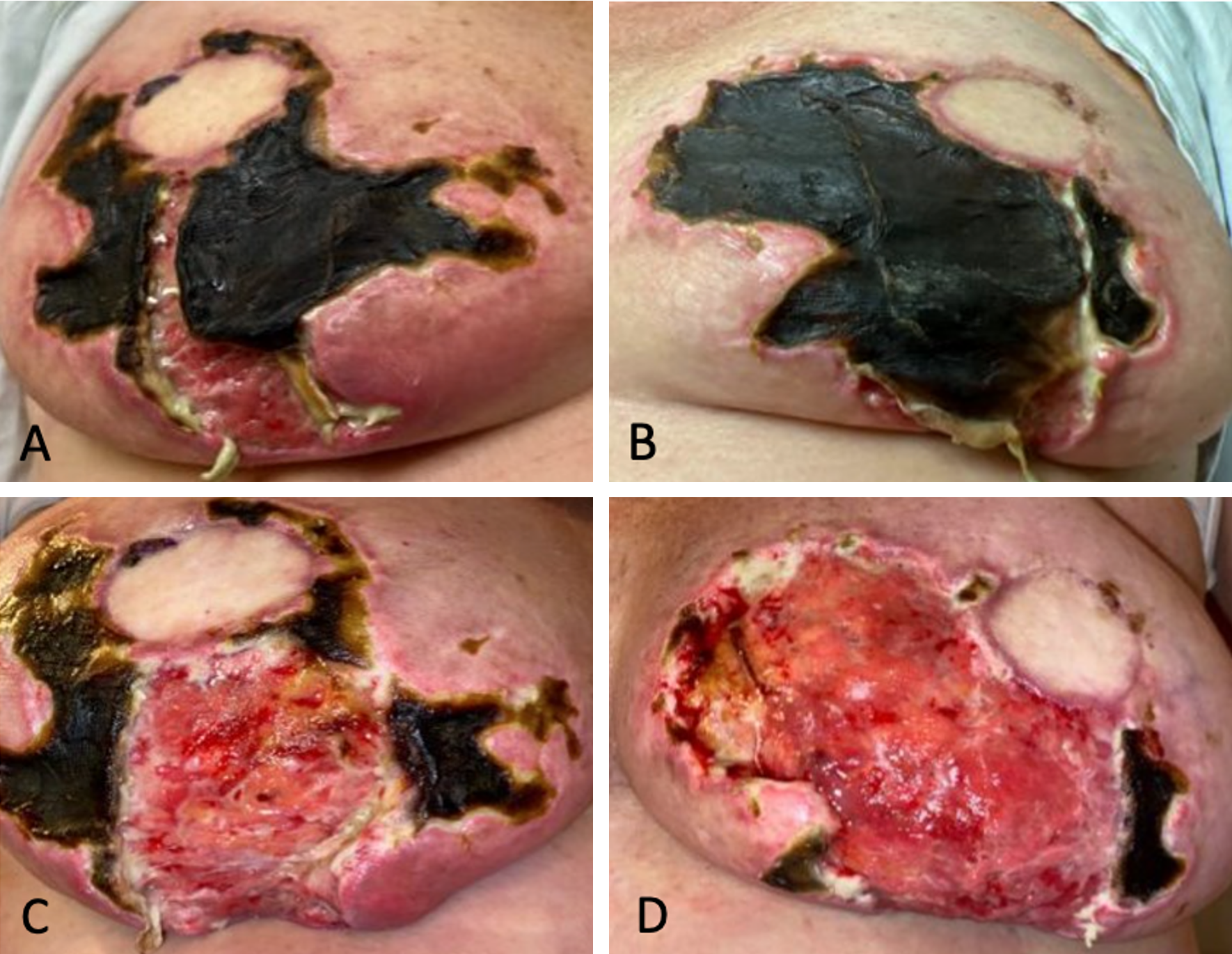
Figure 3. Management and progression of eschars one month post-surgery. (A) Prominent eschar formation on the right breast prior to surgical intervention. (B) Visible eschars on the left breast before debridement. (C) Post-debridement view of the right breast, revealing the underlying tissue. (D) Post-debridement appearance of the left breast, showing the necrotic tissue being removed.
In the following months, she required frequent clinic visits for ongoing debridement of the wounds, particularly where exposed mesh was noted on the right side. Wound care strategies using Xeroform and calcium alginate were employed to facilitate epithelialization. By five months postoperatively, considerable healing had occurred; both breasts and the abdomen had nearly fully recovered (Figure 4).
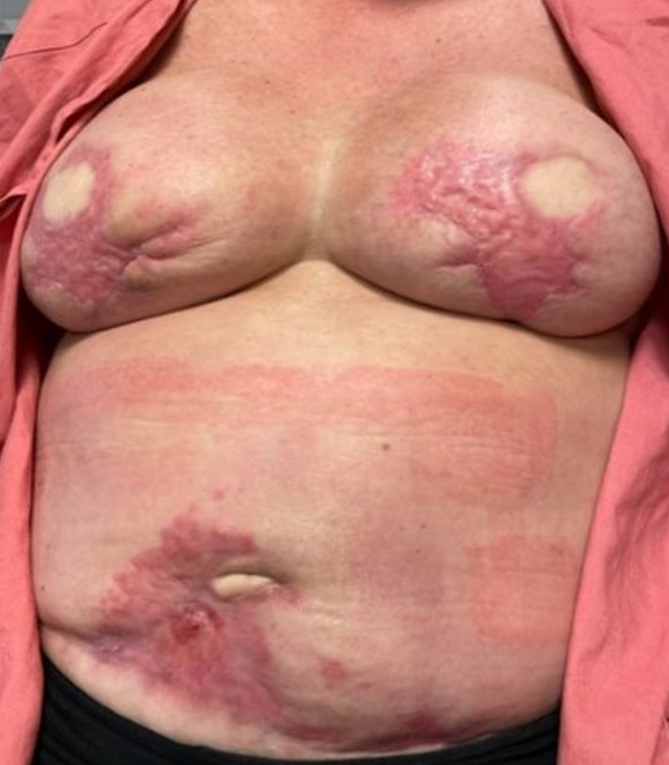
Figure 4. Comprehensive healing with contraction at five months post-surgery. This image displays substantial healing at the bilateral breast and abdominal donor sites. It highlights significant tissue recovery with noticeable contraction, illustrating the effects of the healing process on tissue morphology.
This case study examines a middle-aged woman with a confirmed BRCA gene mutation who exhibited significant delayed wound healing and skin necrosis after undergoing prophylactic mastectomy and breast reconstruction. We hypothesize that this extensive skin necrosis and prolonged wound recovery may be associated with her treatment with fremanezumab for chronic migraines. This hypothesis is supported by three observations: firstly, the degree of delayed wound healing is unusual for uncomplicated free flap reconstruction cases; secondly, this patient lacked conventional risk factors for poor wound healing, such as obesity, smoking, or corticosteroid use; thirdly, the only variable that could influence wound healing was the use of the CGRP antagonist fremanezumab during the perioperative period. These factors suggest a potential link between fremanezumab use and the delayed wound healing observed, although a direct causal relationship has not been definitively established.
Biologics and Surgery: Balancing Risks
The controversy surrounding the practice of stopping biologic medications before cosmetic, elective, or reconstructive surgery is multifaceted, involving the balancing of theoretical risks and practical patient outcomes. The perioperative management of biologic medications necessitates careful consideration of potential complications, such as delayed wound healing or postoperative infections, against the risk of exacerbating the underlying disease if the medication is discontinued.
The 2017 guidelines from the American College of Rheumatology (ACR) and the American Association of Hip and Knee Surgeons (AAHKS) recommend holding biologic medications as close to one dosing cycle as possible before elective procedures [5]. This recommendation is based on the understanding that immunosuppressive medications increase the risk of postoperative infections and complications. This guidance primarily focused on patients undergoing major surgeries like hip or knee arthroplasty.
However, recent studies challenge this approach. Notably, the Postoperative Infection in Inflammatory Bowel Disease (PUCCINI) trial involved 947 patients with inflammatory bowel disease across 17 sites [6]. It investigated the impact of preoperative tumor necrosis factor inhibitor (TNFi) exposure on postoperative infection risks. The study found that neither reported TNFi use within 12 weeks of surgery nor detectable serum TNFi concentrations were independent risk factors for postoperative infections, including surgical site infections. The results of this prospective cohort study suggest that preoperative TNFi treatment does not increase infection risks. Therefore, it should not influence surgical decisions for most patients with inflammatory bowel disease. This provides reassurance for continued use of TNFis close to surgical dates without heightened concerns for postoperative complications.
Additionally, a systematic review conducted by van Duren et al. revealed no significant increase in surgical site infections or delays in wound healing among patients who continued their biologic disease-modifying anti-rheumatic drugs during orthopedic procedures [7]. This analysis also highlighted the limited quality of evidence supporting the perioperative discontinuation of biologic agents, complicating clinical decision-making.
The updated 2022 guidelines from the ACR and AAHKS also reflect these evolving insights [8]. They recommend withholding biologic medications for a dosing cycle before surgery in patients with inflammatory arthritis, but allowing surgery to be scheduled after that dose. For severe cases of systemic lupus erythematosus, continuing biologics is advised, while in less severe cases, withholding biologics is recommended to avoid the risk of organ damage. The updated guidelines incorporate new immunosuppressive medications, highlighting the importance of shared decision-making between doctors and patients.
Discontinuing biologic medications can lead to significant flare-ups of the underlying disease, adversely impacting patient health and quality of life. This risk often outweighs the theoretical postoperative risks, such as delayed wound healing or postoperative infections. Additionally, recent studies indicate minimal perioperative complications with continued biologic use. Consequently, a more individualized approach is advocated, reflecting a shift towards tailored patient care based on the latest evidence.
Overall, the controversy remains due to the need for balancing theoretical risks with practical considerations and the evolving nature of clinical evidence. As new research continues to emerge, it is imperative for guidelines to adapt accordingly, ensuring optimal patient outcomes through personalized care strategies.
Overview of CGRP Monoclonal Antibodies
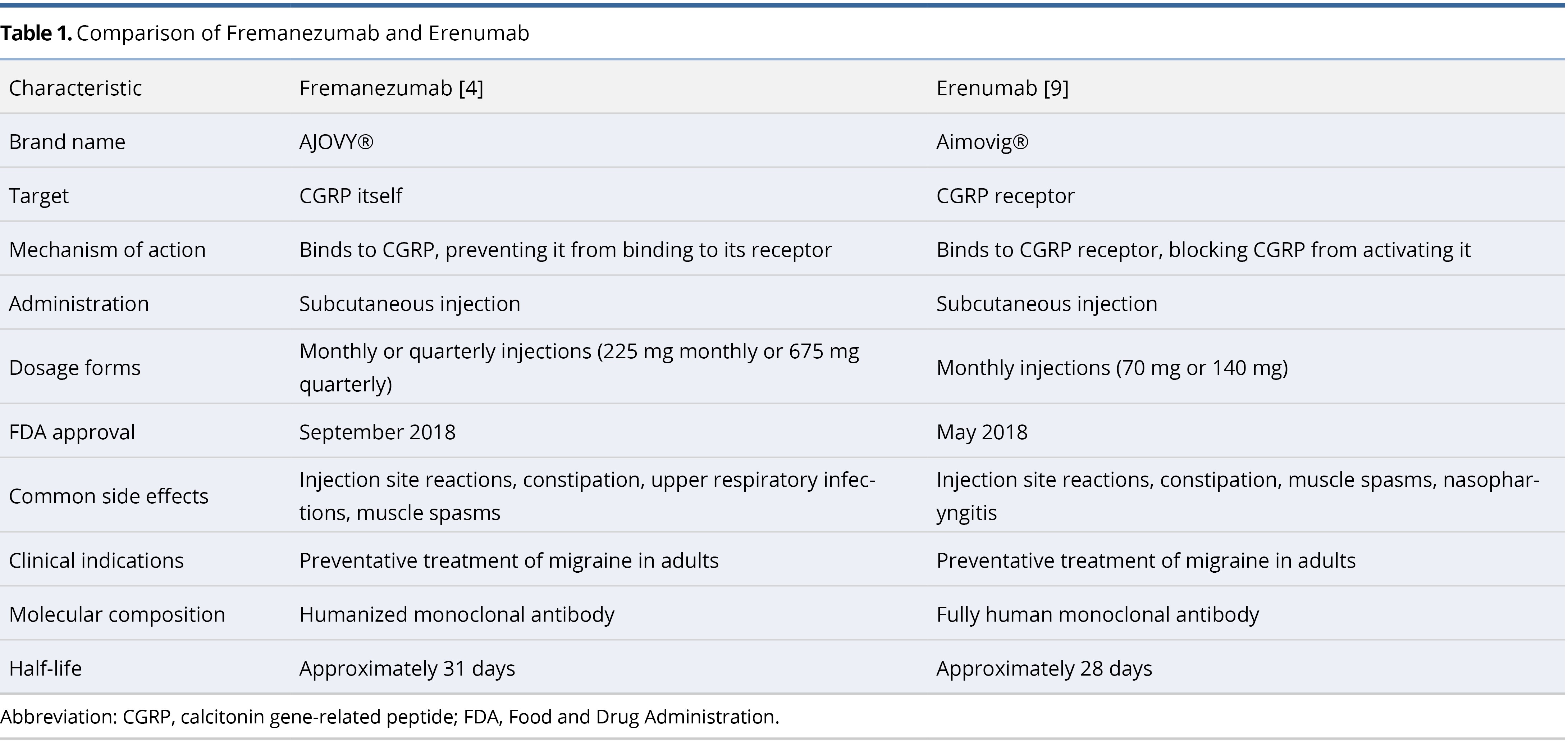
CGRP monoclonal antibodies, specifically erenumab (Aimovig®) [9] and fremanezumab (AJOVY®) [4], have recently emerged as effective and generally well-tolerated alternatives to traditional antimigraine medications. The United States Food and Drug Administration (FDA) approved erenumab in May 2018, followed by fremanezumab in September 2018, thereby setting significant benchmarks in the therapeutic landscape of migraine management [2].
Table 1 provides a comprehensive comparison of fremanezumab and erenumab, detailing their targets, mechanisms of action, administration routes, dosage forms, common side effects, clinical indications, molecular composition, and half-lives. Fremanezumab targets the CGRP molecule directly, while erenumab targets the CGRP receptor. Both agents are administered via subcutaneous injection; fremanezumab offers monthly or quarterly dosing options, whereas erenumab is available in monthly doses. Notably, fremanezumab is a humanized monoclonal antibody containing some non-human components, while erenumab is a fully human monoclonal antibody, which reduces the risk of immune reactions. Fremanezumab has a half-life of approximately 31 days, while erenumab has a half-life of about 28 days.
CGRP Monoclonal Antibodies: Wound Healing Concerns
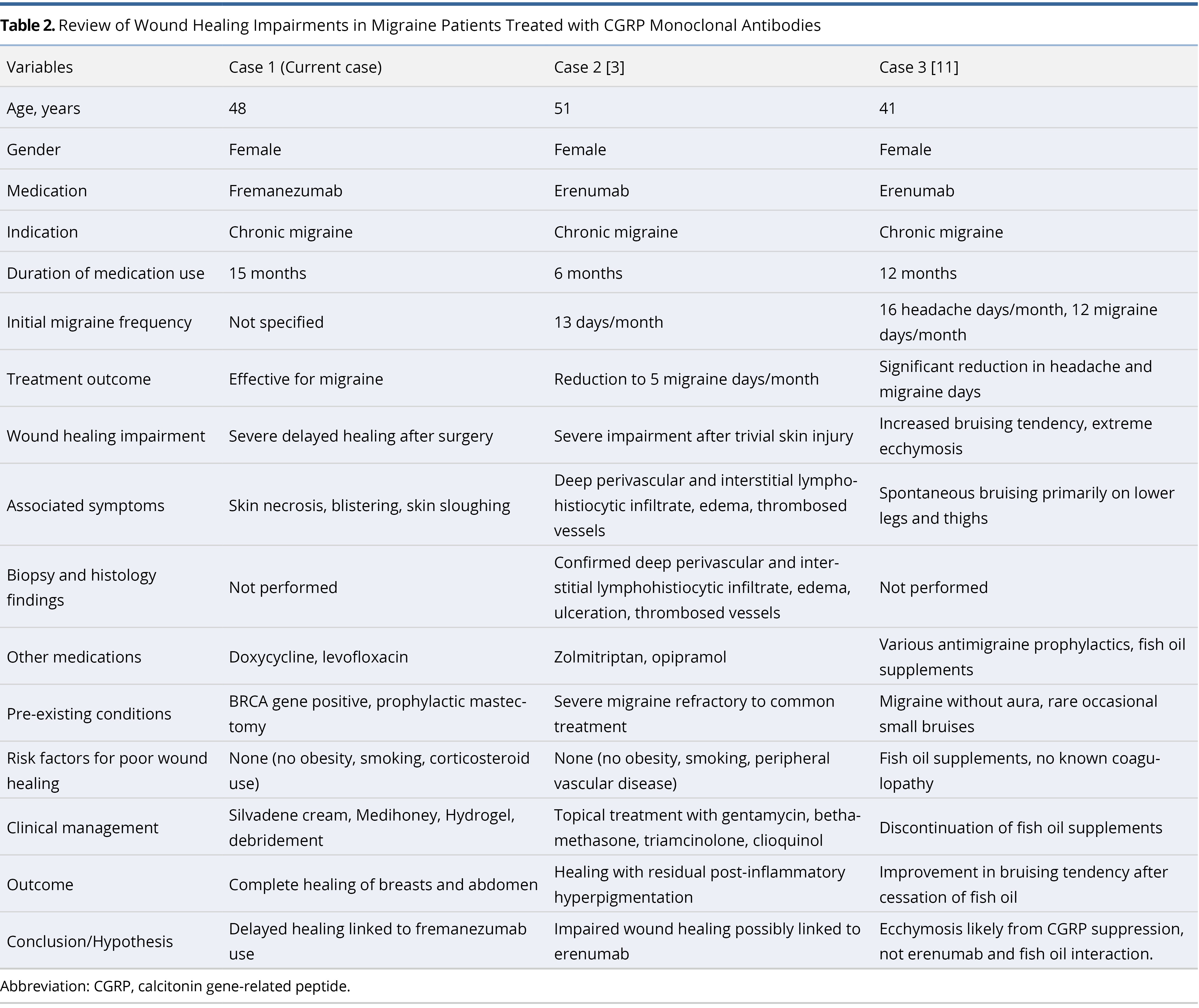
Despite their proven efficacy, these treatments exhibit minimal side effects, typically including constipation, muscle spasms, itching, injection site pain, nasopharyngitis, and upper respiratory tract infections [10]. Notably, the existing literature does not report any instances of impaired wound healing associated with these treatments in surgical settings. However, there have been two reported cases of non-surgical wound healing impairments associated with erenumab [3,11].
The first case involved a 51-year-old woman treated with erenumab for chronic migraines. She developed severe wound healing complications following a minor skin injury, raising concerns about the impact of the drug on wound recovery [3]. The second case described a 41-year-old woman, also treated with erenumab for chronic migraines, who experienced spontaneous bruising primarily on her lower legs and thighs [11]. The hypothesis for the ecchymosis in this patient suggests that CGRP function suppression by erenumab may delay capillary healing, leading to extensive blood leakage and visible bruising. Initially, this bruising was thought to be influenced by the concurrent use of fish oil supplements; however, it is more likely attributed to CGRP suppression rather than a direct interaction between erenumab and fish oil.
Both aforementioned cases are linked to erenumab use [3,11]. Conversely, there are no recorded instances of surgical wound healing complications associated with fremanezumab, particularly in the perioperative period. This report presents the first observed case of a female patient treated with fremanezumab for chronic migraines who experienced delayed wound healing following a free flap breast reconstruction.
Table 2 summarizes these three cases of CGRP monoclonal antibody use in chronic migraine treatment, highlighting wound healing complications linked to CGRP monoclonal antibodies. This comparative analysis underscores the necessity for cautious administration of CGRP monoclonal antibodies, especially in patients undergoing surgery. It also highlights the need for further research into their potential impacts on wound healing.
Labeling Gaps in CGRP Monoclonal Antibody Safety
The package insert for fremanezumab omits warnings about impaired wound healing or elevated infection rates. This omission likely stems from the exclusion of patients with significant cardiovascular or thrombotic conditions from key clinical trials, resulting in a noticeable gap in safety data for these groups [4]. Furthermore, the FDA approvals of erenumab and fremanezumab in 2018 highlight the novelty of CGRP monoclonal antibodies in clinical use [2]. This underscores that the development of comprehensive clinical experience is still ongoing. Consequently, as clinical use expands, it is crucial to monitor and document potential adverse effects rigorously. This approach helps fill existing knowledge gaps and ensures that all safety concerns, especially those related to wound healing and infection rates, are thoroughly addressed in future updates to drug labeling and clinical guidelines.
To address these gaps, this case report aims to supplement the safety data by exploring potential risks in patients undergoing major vascular surgeries. However, it is important to clarify that this report merely presents a clinical observation and does not establish a causal relationship between fremanezumab and delayed postoperative wound healing. The findings are offered as subjective interpretations and are not intended to prompt changes in drug labeling, as other factors could also influence these outcomes.
In light of this rare clinical scenario, it is imperative for healthcare providers to thoroughly assess potential confounding factors that may impact wound healing before administering fremanezumab. These factors include diabetes mellitus, vascular pathologies, persistent infections, conditions necessitating immunosuppression, malnutrition, chronic inflammatory disorders, tobacco use, obesity, psychological stress, and corticosteroid use. Meticulous evaluation of these variables is crucial to minimize any additional risk of delayed wound healing in patients treated with fremanezumab.
Study Limitations
This case report provides valuable insights into potential wound healing issues associated with fremanezumab, yet it has several limitations. The findings are based solely on a single patient’s experience, significantly limiting their generalizability. Additionally, since the potential adverse effects of fremanezumab were not anticipated, the medication was not discontinued to assess symptom reversal. This ongoing use, without a trial of cessation, restricts clear interpretation and may influence the reporting of symptoms, reducing the ability to definitively link fremanezumab to the observed wound healing delays.
This article underscores the need for vigilance when administering CGRP monoclonal antibodies, such as fremanezumab, in perioperative settings. It highlights rare but significant wound healing complications in surgical patients. While the impaired wound healing in the presented case may be linked to fremanezumab, a direct causal relationship is not established. Balancing the risks of delayed wound healing against worsened disease control when using biologic agents for chronic diseases is crucial. These risks should be evaluated on a case-by-case basis.
Received date: May 05, 2024
Accepted date: July 12, 2024
Published date: August 14, 2024
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)’ autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2024 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
The authors describe various patient and breast-related factors that influence surgical outcomes while also addressing some techniques and principles for aesthetic microsurgical reconstruction.
This study examined two surgical techniques commonly used for turbinate reduction (i.e., submucous resection and partial excision) and their associated complications in functional nasal surgery patients. So far, there has been no direct comparison of these two methods with endpoints of epistaxis and nasal congestion. It was found neither technique was statistically significantly different from the other, so both are clinically useful with a low incidence of postoperative epistaxis.
A significant increase in peripheral nerve surgery has occurred in recent years due to improvements in surgical techniques. In most reconstructive procedures, sensory restoration is frequently neglected in preference to restoring motor function. Along with increasing the risk of developing injuries to the body, patients who lose protective sensations are more likely to develop neuropathic pain and depression, which adversely affect their quality of life. As regaining sensory function is important, the study examines a variety of techniques that may be useful for restoring sensory function across various body parts.
This study introduces an advanced tubularized radial artery forearm flap (RAFF) technique, marking an enhancement over traditional methods in addressing complex nasal reconstructions. It integrates functional and aesthetic considerations through a structured, multi-stage reconstruction process, emphasizing the use of tubularized flaps. Key learning points include the detailed crafting of stable nasal passages, strategic use of costal cartilage for robust structural support, and tailored postoperative care with silicone splints. The tubularized RAFF technique not only optimizes patient outcomes and quality of life but also provides plastic surgeons with critical insights to refine their techniques in facial reconstruction. Indispensable for professionals in the field, this article enriches the understanding of sophisticated reconstructive challenges and solutions.
This manuscript showcases an advanced surgical approach for treating malignant giant cell tumor of bone, emphasizing precision and ethical considerations. It leverages innovative pedicled flap technologies, as opposed to free flaps, enhancing limb functionality and patient quality of life. This technique equips surgeons with evidence that tailored surgical strategies can significantly improve outcomes in complex cases. The paper discusses technical challenges and highlights the application of supercharging and superdrainage techniques in limb reconstructions, methods well-established in microsurgery but infrequently used in oncological contexts. These techniques are crucial for optimizing flap viability and ensuring surgical success. Additionally, the manuscript underscores the profound impact of these advancements on patient lives, offering hope and showcasing tangible benefits. This narrative, blending scientific analysis with patient stories, enriches the understanding of limb reconstruction innovations in oncological surgery, making it invaluable for surgeons.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
This systematic review and meta-analysis provide a pragmatic evaluation of drain-free versus drain-based DIEP flap techniques for breast reconstruction, challenging the traditional reliance on drainage. By analyzing postoperative outcomes, the study highlights the potential for refining surgical strategies to enhance patient comfort and recovery without compromising safety. The findings offer a neutral perspective, suggesting that clinical practice may not necessarily depend on the use of drains. This revelation prompts medical professionals to reassess existing surgical approaches and may catalyze a paradigm shift in postoperative care. Presented with clear narrative and rigorous data analysis, the article encourages readers to consider the broader implications of surgical innovations on patient care protocols.
This study meticulously investigates the potential adverse effects of fremanezumab on wound healing, a rarely documented concern for patients receiving this CGRP receptor antibody for migraine prevention. Highlighting a case of delayed wound healing post-surgery, the manuscript emphasizes the necessity for increased clinical awareness and further research into the mechanisms underlying this complication. This crucial insight challenges the current understanding of fremanezumab's safety profile and calls for enhanced patient monitoring and risk assessment. The article is nearly ready for publication, requiring only the resolution of a few minor issues.
The article "Impaired Wound Healing in a Patient on Fremanezumab for Chronic Migraine Following Immediate Free Flap Breast Reconstruction: A Case Report" significantly contributes to the understanding of the complex interplay between biologic therapies and surgical outcomes. By meticulously detailing a unique case of severe wound healing complications in a patient treated with fremanezumab, the authors illuminate a critical and previously underreported potential side effect of CGRP monoclonal antibodies. The thorough documentation and insightful discussion provided not only underscore the need for heightened awareness among clinicians but also pave the way for further research in this domain. This article exhibits expertise in its execution and merits publication, provided that a particular aspect is addressed and resolved.
I reviewed the article "Impaired Wound Healing in a Patient on Fremanezumab for Chronic Migraine Following Immediate Free Flap Breast Reconstruction: A Case Report" and found it to be an insightful exploration into the adverse effects of CGRP monoclonal antibodies on surgical recovery. This case report is pivotal, highlighting a rarely documented risk of delayed wound healing and offering valuable insights into the management of patients undergoing surgical procedures while on biologic therapy. However, the article may face acceptance challenges due to its reliance on a single case, the need for a more extensive review of the relevant literature, and the lack of comparative data. Addressing these points with additional context and supporting evidence will significantly enhance the manuscript's impact.
The manuscript has been notably enhanced, now including a comparative table (Table 1) that elucidates the distinct case of delayed wound healing associated with fremanezumab following breast reconstruction. Additionally, it delineates the pharmacological distinctions between fremanezumab and erenumab (Table 2). To prepare the manuscript for publication, the authors are advised to augment the discussion of study limitations. This should address the concerns raised by Reviewer 2, by conducting a comprehensive analysis of potential confounding factors that may affect wound healing in patients treated with fremanezumab. Such an expansion will increase the manuscript’s depth and scientific rigor. An example for consideration is provided below:

RevisionThe authors have expanded the study limitations section to address potential confounders such as diabetes, vascular disorders, and other factors affecting wound healing in patients treated with fremanezumab. This revision enhances the manuscript's depth and scientific integrity, addressing the editor's and Reviewer 2's concerns effectively.
The authors have effectively addressed my previous comments. They have refined the abstract to concisely present the clinical question, key findings, and significance of the report, thus enhancing its accessibility in electronic databases. This revision considerably advances our understanding of the potential adverse effects of fremanezumab on wound healing. The thorough documentation and insightful discussion highlight the need for increased clinical vigilance and further investigation. Given these comprehensive revisions and the significance of the findings, I recommend the article for publication.
The authors have meticulously detailed the medical background of the patient, a 48-year-old female with chronic migraine managed by fremanezumab, who underwent breast reconstruction following a positive BRCA gene test. This clarification supports their hypothesis that the delayed wound healing observed in this case may be primarily attributable to fremanezumab administration. The authors have satisfactorily addressed my concerns, and I recommend the manuscript for acceptance in the journal.
Honig SE, Li SS, Broach RB, Serletti JM. Impaired wound healing following free flap breast reconstruction in a patient treated with fremanezumab: A case report and implications for perioperative management. Int Microsurg J 2024;8(1):5. https://doi.org/10.24983/scitemed.imj.2024.00188