Robotic-assisted surgery has revolutionized the surgical world, introducing innovative methods to reduce invasiveness across a variety of procedures. Despite its promise, the adoption of robotic-assisted techniques in microsurgery has been gradual. Many microsurgical procedures traditionally rely on open approaches and demand a level of technical skill that exceeds the current capabilities of robotic systems. The robotic-assisted deep inferior epigastric perforator (DIEP) flap for breast reconstruction exemplifies a pioneering application of robotic technology that enhances the "gold standard" for flap-based breast reconstruction. This technique enables microsurgeons to harvest the pedicle of the abdominal flap with a significantly shorter fascial incision. It is hypothesized that minimizing the fascial incision length could mitigate donor site morbidity and related complications, such as core weakening, pain, and the risk of fascial bulge or hernia. This manuscript delves into the robotic-assisted DIEP flap, elaborating on the operative technique and sharing critical surgical insights necessary for successful implementation. Furthermore, it reviews the pertinent literature, underscoring both the successes and potential areas for enhancement of the robotic-assisted DIEP flap. This comprehensive examination showcases the current advancements and sets the stage for future innovations in the field of robotic-assisted microsurgery.
Robotic-assisted surgery (RAS) was first conceptualized in the 1980s by Scott Fisher at the National Aeronautics and Space Administration (NASA) and Joseph Rosen, a plastic surgeon at Stanford University [1]. Originating as a derivative of laparoscopic surgery, RAS aims to improve surgical outcomes through minimally invasive approaches, thereby reducing human error [2]. Since the introduction of early robotic equipment such as the Programmable Universal Machine for Assembly (PUMA) Arm and RoboDoc, designed for neurologic and orthopedic surgery respectively, to the advent of the da Vinci® System, the field of RAS has experienced exponential growth over the past decades [1,2].
The da Vinci® System, which consists of a surgeon’s console equipped with cameras for each eye, a patient trolley with four articulated arms, and an advanced imaging system, was the first surgical robot to receive FDA approval in 2000 [2,3]. It has been widely adopted across various medical subspecialties, including urology, gynecology, otolaryngology, cardiothoracic, and abdominal surgery [4–6]. Research on outcomes in these fields has underscored the significant benefits of RAS for both patients and providers.
RAS has been shown to minimize morbidity and mortality by reducing risks associated with surgical tremor and fatigue, providing seven degrees of motion, and offering three-dimensional vision, thereby enhancing the surgeon’s dexterity and the visualization of the surgical field [6]. Given that the initial surgical robot was developed with clinical input from Joseph Rosen, a plastic surgeon, specifically to enhance neurovascular anastomoses in hand surgery, RAS has consistently demonstrated significant potential within the field of plastic surgery [1]. Its swift adoption in otolaryngology, particularly through transoral robotic surgery, exemplifies one way RAS has penetrated plastic surgery [7]. Research on transoral robotic surgery has shown it facilitates easier dissection, reduces damage to adjacent anatomy, and improves both visualization and ergonomics for surgeons [7–9].
Similarly, the success of RAS in oncology has spurred the development of robotic techniques for nipple sparing mastectomy [9–13]. Studies have revealed that when RAS is applied to the harvesting of flaps, particularly the latissimus dorsi and deep inferior epigastric perforator (DIEP) flaps, it enhances visualization and reduces surgical complications and scarring [13–16]. Additionally, RAS has highlighted the benefits of tremor filtration and motion scaling in creating effective and successful anastomoses [13,17]. Head and neck and breast reconstructions are two areas where RAS can greatly improve both the surgical experience and outcomes, offering substantial advancements in plastic surgery.
The authors report on a 63-year-old postmenopausal female patient with a documented history of moderately differentiated invasive ductal carcinoma in the left breast. The tumor tested positive for estrogen and progesterone receptors (ER+/PR+), and negative for the human epidermal growth factor receptor 2 (HER2-). It was staged as pT2N0 under the tumor, node, metastasis (TNM) classification system and classified as Stage 1B according to the American Joint Committee on Cancer (AJCC) standards.
She underwent a skin-sparing mastectomy of the left breast and prepectoral tissue expander reconstruction with an acellular dermal matrix. Neoadjuvant anastrozole was required, but adjuvant radiation therapy was not administered. Following the completion of her oncologic care, she expressed concerns about deformity, asymmetry, and mastodynia in her reconstructed breast.
Consequently, she showed interest in autologous breast reconstruction and decided to undergo a delayed microsurgical reconstruction of the left breast using a robotic-assisted right DIEP flap. This decision was informed by a detailed discussion of her options and preferences, taking into account her surgical history and insights from magnetic resonance angiogram scans.
For the robotic-assisted DIEP approach, preoperative imaging is essential for perforator mapping, selection, and assessment of the intramuscular course of the pedicle. The ideal candidate typically displays a single perforator or two grouped perforators in close proximity with a short intramuscular course. Analysis of the length of the intramuscular course is crucial for determining candidacy, as the fascial incision must extend the entire length of the pedicle’s intramuscular course at a minimum. For this patient, the magnetic resonance angiogram revealed two notably large perforators, making her a suitable candidate for this advanced surgical technique.
Preoperative Marking and Initial Dissection
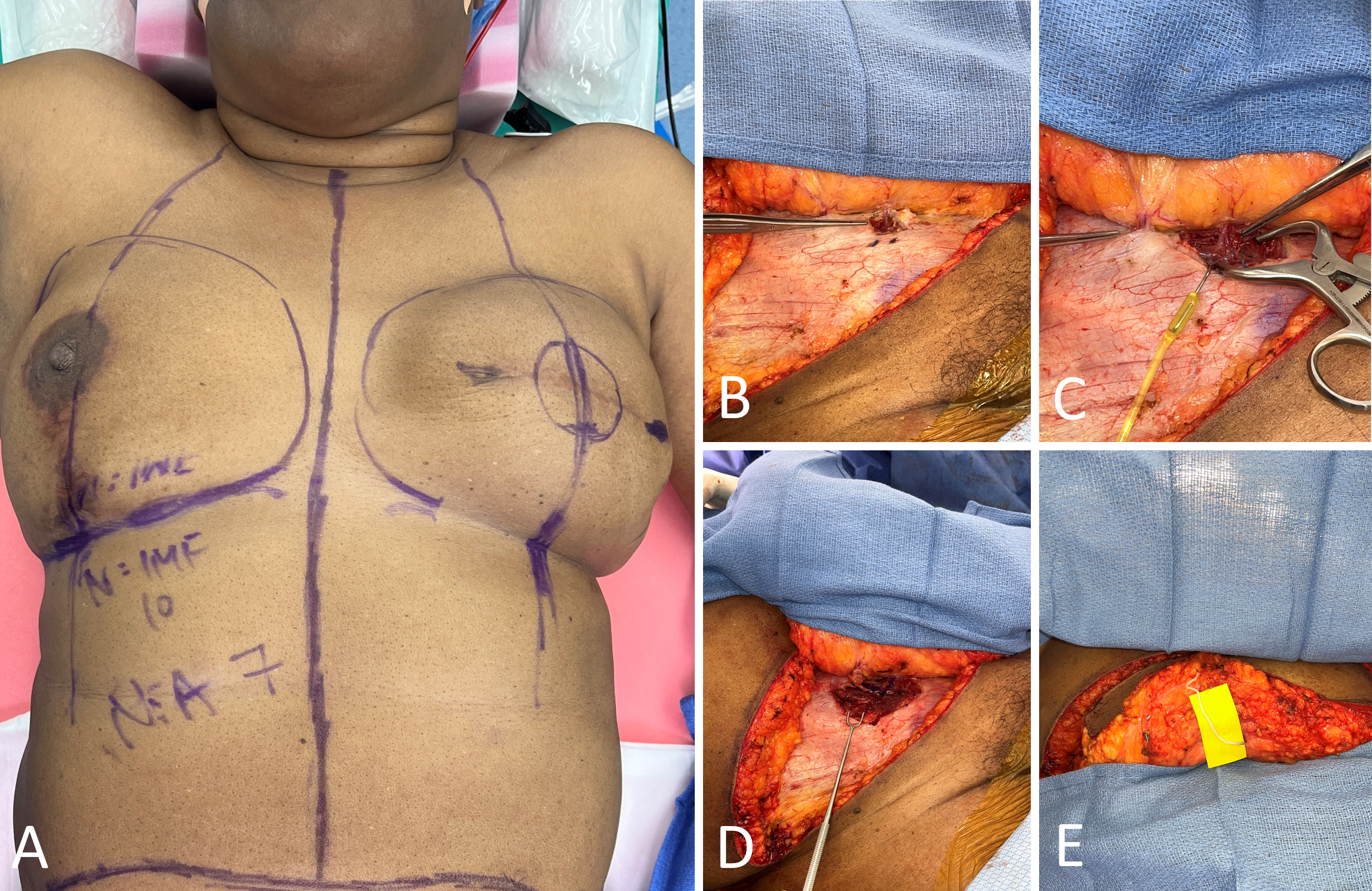
The donor and recipient sites were marked preoperatively while the patient was in the upright position (Figure 1A). The robotic-assisted DIEP flap technique began with the elevation of the abdominal flap, with dissection performed down to the anterior rectus fascia, similarly to the conventional open approach. The right abdominal donor site flap was elevated centrally from lateral to medial, with direct undermining based on the selected perforators identified by preoperative imaging (Figure 1B). Lateral perforators were clipped and ligated until two large medial row perforators were identified.
Once the pre-selected perforators were exposed, the anterior rectus fascia was minimally incised. The pedicle was then traced to the retromuscular position, and at this point, the remainder of the dissection was performed submuscularly (Figure 1C). The vertical length of the fascial incision was limited to the region through which the pedicle runs intramuscularly. In this case, a separate perforator was in close proximity to the perforator of interest along the same medial row, and therefore the fascial incision was extended to expose both perforators (Figure 1D). Despite this extension, the overall length of the incision did not preclude the robotic-assisted approach. Notably, a nerve graft may be performed for coaptation after the DIEP flap is harvested (Figure 1E).

Figure 1. Preoperative marking and initial dissection. (A) Preoperative markings for a 63-year-old female with a history of left breast cancer, scheduled for delayed left breast microsurgical reconstruction utilizing a robotic-assisted right deep inferior epigastric artery perforator (DIEP) flap. (B) The targeted perforator is successfully identified and exposed, facilitated by preoperative magnetic resonance angiography. (C) The procedure identifies a single dominant perforator, optimal for the robotic-assisted DIEP flap harvest approach. Notably, an additional perforator is observed in proximity to the primary target along the same medial row. (D) A minimal extension of the fascial incision is performed to expose both perforators. Despite this extension, the incision's length remains conducive to the robotic-assisted approach. Once exposed, meticulous dissection is conducted following the pedicle to the submuscular plane. (E) A nerve graft is executed for coaptation following the harvesting of the DIEP flap.
Securing and Preparing the DIEP Flap
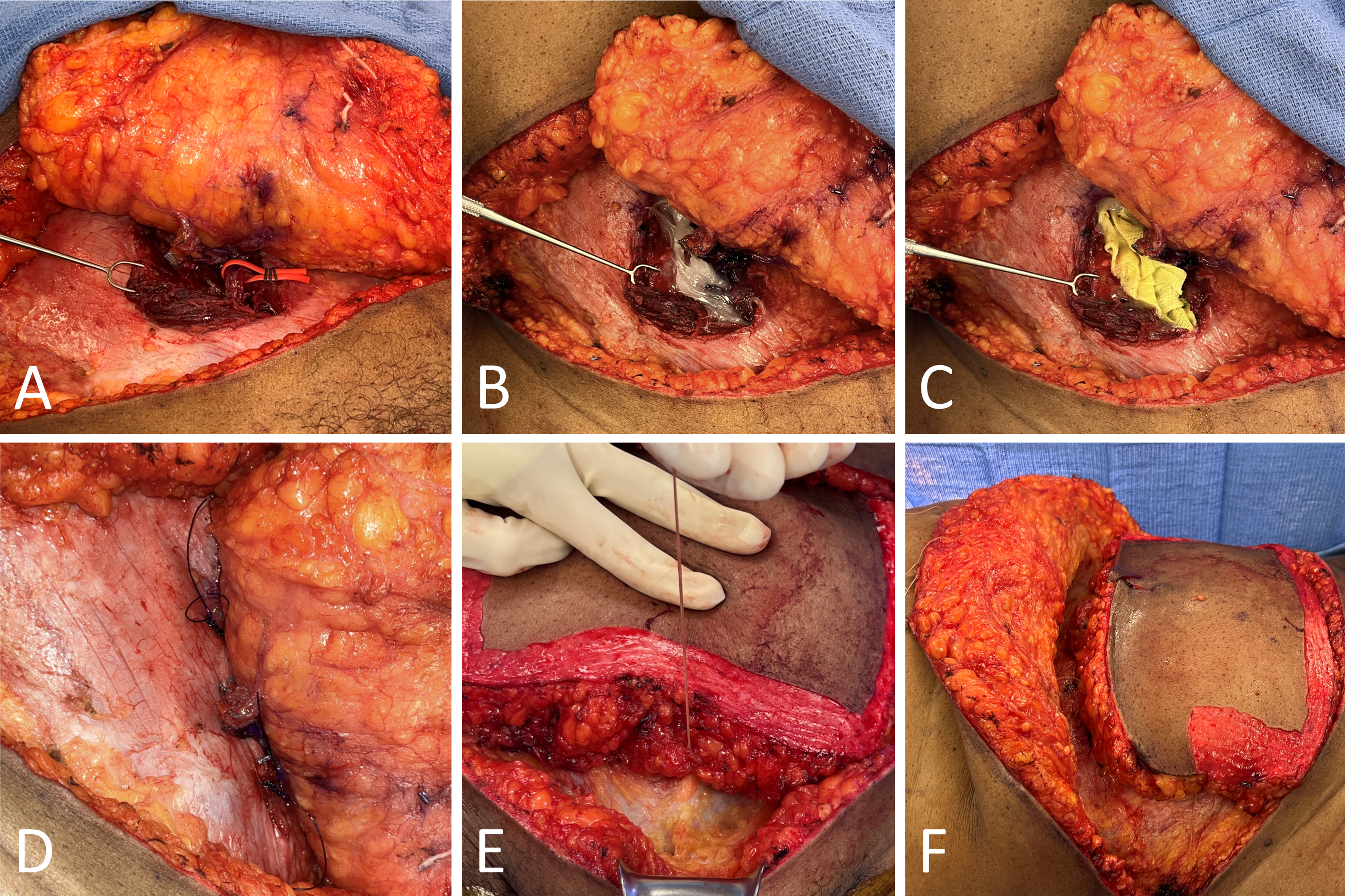
Once the pedicle was dissected posterior to the rectus muscle, a vessel loop was loosely secured around the pedicle from the open, anterior exposure (Figure 2A). The loop was then channeled and nestled in the submuscular plane to facilitate robotic-assisted retrieval of the vessel loop intraperitoneally. To allow insufflation of the abdomen, Bacitracin ointment covered with Xeroform gauze was used as a temporary seal to minimize the escape of gas from the fascial defect. This seal was also temporarily reinforced with sutures (Figures 2B–D).
To ensure that the pedicle was not compromised after establishing a seal, the skin paddle of the abdominal flap was checked for adequate perfusion through assessment of color, capillary refill, and audible Doppler signal. The entire left hemi-abdominal flap was elevated prior to robotic-assisted dissection so that the flap was solely reliant on its right two perforators. After an adequate seal was established and the left hemi-abdominal flap was elevated, Size 0 Vicryl sutures were used to secure the DIEP flap and robotic-assisted access into the peritoneal cavity was performed (Figures 2E–F).

Figure 2. Securing and preparing the deep inferior epigastric perforator (DIEP) flap. (A) A red vessel loop is circumferentially secured around the pedicle and gently tunneled into the submuscular plane. Intra-abdominally, the vessel loop aids in pedicle retrieval and minimizes the risk of pedicle injury during robotic-assisted dissection. To mitigate gas leakage from the external defect during insufflation, Bacitracin ointment (B), Xeroform gauze dressing (C), and two size 0 polydioxanone (PDS) sutures (D) are applied sequentially. The sutures are strategically placed above and below the perforator. (E) Size 0 Vicryl sutures are employed to secure the DIEP flap during insufflation. (F) Size 0 Vicryl sutures are also utilized to reflect the superior flap, allowing for optimal robotic docking.
Bilateral DIEP Flap Considerations
In bilateral DIEP flap procedures, it is crucial to preserve the superior continuation of the perforator in the hemi-abdominal flap that is scheduled for secondary recipient site microsurgery. Alternatively, a separate perforator from the second transposed hemi-abdominal flap can be exposed, preserved, and maintained in situ based on the opposite row to ensure adequate perfusion of the flap. This is done after the pedicle of the primary perforator of interest has been clipped and ligated with robotic assistance intra-abdominally. The second perforator or the superior continuation of the perforator in the second hemi-abdominal flap is ligated once the second flap is prepared for microsurgical anastomosis. This careful preservation and management of perforators are essential to ensure the viability and successful integration of both flaps during the bilateral DIEP flap procedure.
Robotic DIEP Flap Procedure
At our institution, at this point in the procedure, a general surgeon with advanced skills in robotic techniques assumes the lead. The interdisciplinary nature of our team, which includes general surgeons, enables us to safely dissect the vascular pedicle robotically.
An open Hasson entry technique was utilized to access the peritoneal cavity, though a Veress needle could alternatively be used. After inserting an AirSeal port (CONMED, Utica, NY, USA), pneumoperitoneum was established at a pressure of 10–15 mmHg. A camera was then placed through the insufflation port to assist in the insertion of three additional 8-mm robotic ports. These ports were inserted above the umbilicus through the fascia at the level of the epigastrium, in a manner similar to the robotic transabdominal preperitoneal (rTAPP) repair for inguinal hernias (Figure 3A).
Alternatively, the contralateral ports relative to the targeted DIEP flap may be placed lateral to the semilunar line and along an imaginary line connecting the anterior superior iliac spine and the anterior axillary line, with the middle port positioned between the anterior superior iliac spine and the anterior axillary line. This approach necessitates undocking of the robot when switching to the contralateral side during a bilateral DIEP procedure, which reduces overall efficiency compared to the previously described approach that allows for single docking when harvesting both pedicles.
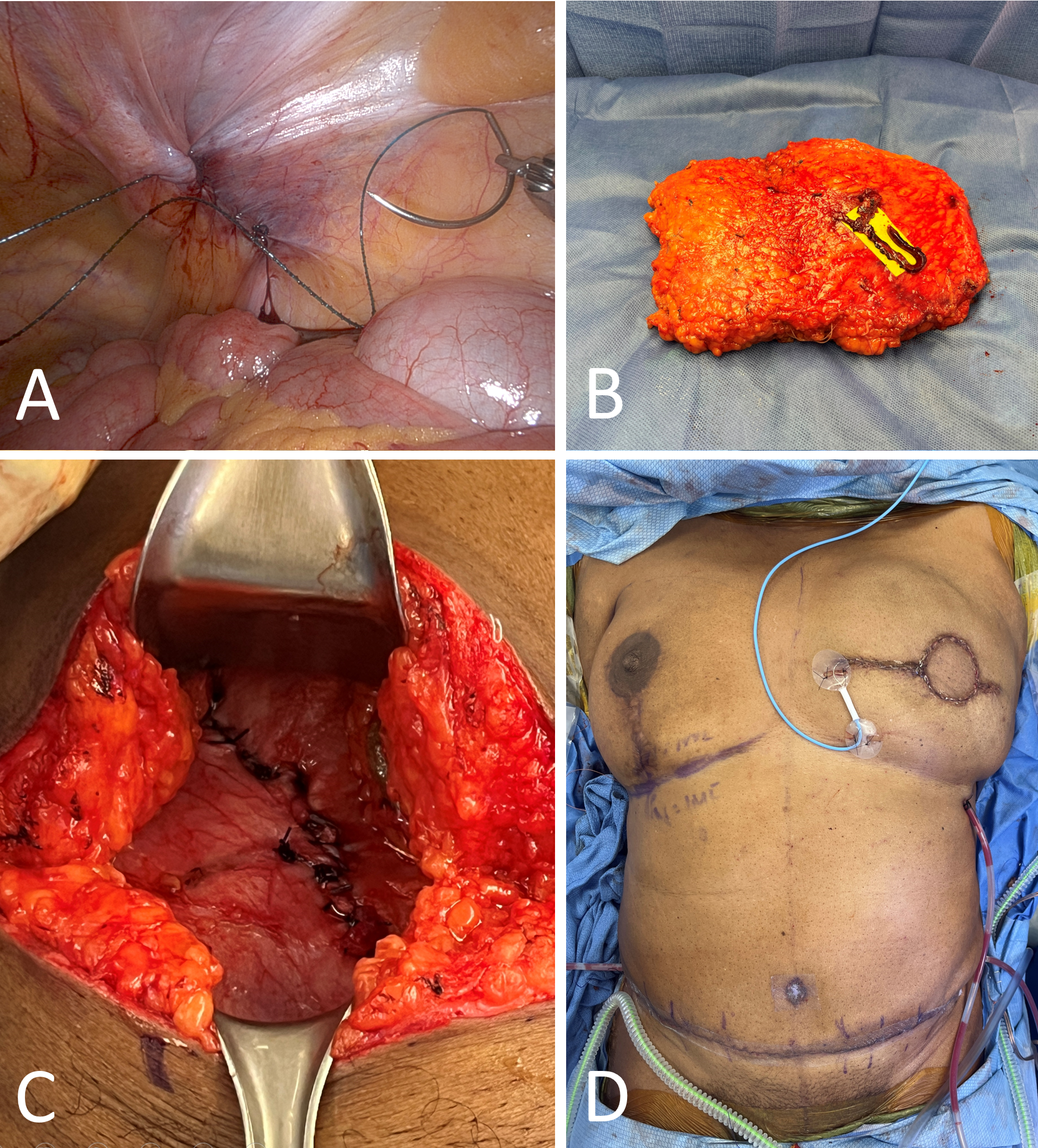
Monopolar scissors and fenestrated bipolar graspers initiated the intraperitoneal dissection of the pedicle (Figure 3B). The peritoneum was incised lateral to the lateral umbilical fold until the previously positioned vessel loop was visualized and retrieved. This vessel loop was then manipulated to aid in the dissection of the pedicle up to its proximal origin at the level of the external iliac vessels. Microclips and bipolar graspers were employed to sever all contributing side branches until the pedicle was substantially freed (Figure 3C).
Following thorough dissection, the pedicle was clipped, cut, and detached from the external iliac vessels (Figures 3D–E). The pedicle was then divided distally and completely removed through the external fascial opening. A detailed video supplement demonstrating the robotic-assisted extraction of the vascular pedicle in a DIEP flap procedure is available at https://doi.org/10.24983/scitemed.imj.2024.00185. This video clearly illustrates the removal process from the intra-abdominal cavity.

Figure 3. Robotic-assisted deep inferior epigastric perforator (DIEP) flap procedure. (A) Port placement mimics the approach used in robotic transabdominal preperitoneal (rTAPP) repair for inguinal hernia, with pneumoperitoneum established at a pressure of 10–15 mmHg. (B) Monopolar scissors and a fenestrated bipolar grasper are utilized to incise the peritoneum. The red vessel loop is retrieved intra-abdominally to facilitate optimal pedicle dissection. (C) All contributing side branches are either clipped using microclips or ligated with the bipolar device to ensure clear pedicle dissection. (D) Microclips are applied to the pedicle near its proximal origin at the level of the external iliac vessels. (E) After clipping, monopolar scissors are used to cut the pedicle at its base.
Video Following robotic-assisted ligation, the vascular pedicle of the deep inferior epigastric perforator flap is externally extracted and removed from the intra-abdominal cavity.
Closure and Final Steps
Robotic-assisted closure of the posterior rectus sheath was carried out, followed by the undocking of the robotic arms from the patient (Figure 4A). The pneumoperitoneum was then decompressed, and the port sites were closed using figure-of-eight sutures. The operation proceeded as a traditional DIEP procedure with the closure of the small external fascial defect after the flap had been removed and readied for microsurgical anastomosis (Figures 4B–D).

Figure 4. Closure and final steps. (A) A barbed suture is used to robotically close the posterior rectus sheath in a running fashion. (B) The isolated abdominal flap after the pedicle is completely ligated and removed through the external fascial defect. (C) Closure of the external fascial defect shows a defect length confined to less than 5 cm. (D) Immediate postoperative result.
To fully appreciate the current applicability and future potential of robotic-assisted DIEP flap breast reconstruction, it is crucial to understand its historical context. Autologous breast reconstruction has gained popularity, as numerous studies have shown it leads to higher patient satisfaction, fewer long-term complications, and superior aesthetic outcomes compared to implant-based reconstructions [18,19]. The DIEP flap has become the “gold standard” for breast reconstruction. While other flaps, such as thigh and trunk-based flaps, are also viable options, the DIEP flap is particularly advantageous due to the abundant availability of donor tissue in the abdominal area [20].
The main drawback of the DIEP flap is that the surgeon must create a lengthy fascial incision to harvest the pedicle. These fascial openings sometimes extend below the arcuate line, leaving patients more susceptible to significant postoperative pain, abdominal weakening, fascial bulge, or hernia [21,22]. Dr. Jesse Selber pioneered the robotic-assisted DIEP flap in the last decade to offer the benefits of autologous breast reconstruction with the DIEP flap while minimizing invasiveness [14]. With the robotic-assisted DIEP flap technique, the fascial incision only needs to extend as long as the pedicle’s intramuscular course. The remainder of the pedicle is harvested intra-abdominally using robotic assistance. This approach significantly reduces the length of the fascial incision, theoretically lowering the risk for significant pain and the aforementioned donor site complications.
While the robotic-assisted DIEP flap represents a relatively new advancement in microsurgical breast reconstruction, it has already shown promising clinical outcomes. In 2022, Selber and colleagues published a preliminary case series involving 21 patients who underwent robotic-assisted DIEP flap breast reconstruction [12]. This cohort had a mean fascial incision length of 3.6 ± 1.6 cm, significantly shorter than the traditional 13 cm incision associated with the standard DIEP technique. The mean pedicle length for this group was 13.3 ± 1 cm, and none of the patients developed bulges or hernias. Although the study’s small sample size precludes definitive conclusions about complication rates, the reduction in fascial incision length without compromising pedicle length is notable.
Other institutions have also documented early success with the robotic-assisted DIEP flap technique. Lee et al. published an article reporting significantly lower levels of postoperative pain in patients who underwent robotic-assisted DIEP flap breast reconstruction compared to those who had traditional DIEP flap surgery [16]. Wittesale et al. conducted a retrospective review of outcomes at their institution and found no flap failures or intra-abdominal complications among 10 patients who received robotic-assisted DIEP flaps. They noted a steep learning curve associated with the robotic-assisted DIEP technique; although it did not impact the success of the surgeries, the operative time was significantly longer than that required for traditional DIEP flaps [23]. Furthermore, Daar et al. reported on a series involving four patients who underwent robotic-assisted DIEP flaps, with none experiencing flap loss or abdominal site complications [24]. While clinical outcome studies are still limited, initial reports consistently affirm the clinical viability and safety of the robotic-assisted DIEP flap technique, showing promising signs of improved postoperative outcomes.
Limitations in Patient Eligibility
The promising early success of robotic-assisted DIEP flap breast reconstruction is not without its limitations. A significant limitation of the current technique is that not all patients are ideal candidates. As previously discussed, if preoperative imaging shows a patient’s vascular anatomy to have a long intramuscular course, a longer fascial incision is required, reducing the benefits of the robotic-assisted technique. Currently, there is no method to harvest the pedicle with an incision shorter than the length of the pedicle’s intramuscular course [25]. This restricts the number of patients who can benefit from this advanced surgical method.
Technical Challenges and Port Setup
Additionally, the technology used in RAS involves a complex port setup. Multiple port sites are currently required, and for some bilateral procedures, the robotic system must be repositioned for each side [14]. Efficiency and invasiveness could be improved by reducing the number of necessary ports and eliminating the need for repositioning. The technical limitations of the robotic system can partially be attributed to its relatively new application in harvesting robotic-assisted DIEP flaps. Although it has been demonstrated that RAS can safely provide more minimally invasive breast reconstructions, the systems were not originally designed with this specific technique in mind. As the robotic-assisted DIEP flap, and more broadly, robotic microsurgical procedures, continue to be recognized for their superior outcomes, it is likely that robotic surgical systems will evolve to allow more seamless technical integration.
TAPP Versus TEP Approaches
There are two techniques that can be used for the robotic-assisted DIEP flap: the transabdominal preperitoneal (TAPP) approach and the totally extraperitoneal (TEP) approach. This paper focuses on the TAPP approach, with which we have experienced significant initial success at our institution. The TAPP approach offers several advantages over the TEP approach, including shorter operative times and a simpler learning curve. However, it is crucial to discuss the drawbacks of the TAPP approach, notably its more invasive nature due to the need to enter the intraabdominal cavity. Additionally, this technique presents a higher barrier to entry, particularly for plastic surgeons who are not proficient in intraabdominal procedures.
Conversely, the TEP approach provides completely extraperitoneal access to the vascular pedicle. Although the advantages of the TEP approach are well-documented, its steep learning curve and longer operative times have limited its widespread adoption for robotic-assisted DIEP flap harvest [26]. Manrique et al. conducted a cadaveric study comparing the TAPP and TEP approaches, validating the feasibility of both methods and confirming their theoretical advantages and limitations [27]. Further studies are essential to determine the most advantageous approach, enabling surgeons to refine and master a preferred technique.
Cost and Operative Time Considerations
The most significant drawback of the robotic-assisted DIEP flap technique is the increased cost and operative time. Firstly, the upfront cost of a robotic surgical system is substantial, and for institutions that do not already own one, this can be a considerable barrier [28,29]. Even if an institution already has a robotic surgical system or the upfront cost is not an issue, the robotic-assisted DIEP flap requires significantly longer operative times compared to the traditional DIEP flap. This increase in operative time results in higher hospital and anesthesia fees; however, reimbursement rates for robotic-assisted and traditional DIEP flaps are the same [30].
While studies are being conducted to analyze the overall cost-effectiveness of the robotic-assisted DIEP flap technique, there remains uncertainty as to whether the initial investment in operative time and cost is justified in the long term [31,32]. However, analysis of cost-effectiveness in other specialties suggests promising results. For example, Leow et al. reported that robotic-assisted prostatectomies could reduce overall hospital expenses compared to traditional radical open prostatectomies by decreasing time spent in the intensive care unit and shortening hospital stays [33]. Furthermore, Rodrigues Martins et al. showed that experience plays a significant role in improving cost-effectiveness, providing further incentive to develop effective multidisciplinary training protocols [34].
Advancing Robotic DIEP Flap Efficiency
For the robotic-assisted DIEP flap technique to continue gaining popularity and acceptance, refining the technique and preparing the next generation of surgeons are crucial. Enhancing the port setup required for robotically harvesting the pedicle of the DIEP flap could significantly improve the efficiency of the procedure. Currently, the technique requires multiple robotic port sites and frequent repositioning of the surgical robot in bilateral cases.
Further research and validation of single-port techniques are essential. Advancements in robotic technology that enable bilateral pedicle harvesting without repositioning could make surgeries less invasive. These innovations would also significantly reduce operative times, improving overall surgical efficiency and patient outcomes. While there are promising studies indicating major improvements in robotic surgical systems [6,35], these advancements have yet to be applied and validated for the robotic-assisted DIEP flap technique. Moreover, robotic surgical systems designed for microsurgery are rapidly evolving. As these systems with enhanced technical capabilities become more widely available, it is conceivable that procedures currently unsuitable for RAS, such as vascular anastomosis and nerve reinnervation, could also be performed robotically.
To address the issues of increased operative time and costs associated with the robotic-assisted DIEP flap technique, enhancing the operative efficiency of surgeons is key. Research indicates that even minor improvements to the operating room setup and coordination can significantly boost surgical efficiency [36,37]. Additionally, the noted steep learning curve associated with this technique underscores the importance of surgeons gaining proficiency and comfort with the necessary robotic technology and procedures [12,23]. Dr. Selber has advocated for the expansion of plastic surgery residency curriculums to include training in RAS [38], highlighting the critical need for hands-on experience.
Interdisciplinary Programs for Training
The development of interdisciplinary programs is vital. These initiatives should enable general surgeons with advanced robotic training to instruct residents, fellows, and practicing plastic surgeons. Such programs could drastically accelerate the learning process, enabling surgeons to achieve peak operational efficiency more swiftly. Furthermore, by educating and training the next generation of surgeons in robotic techniques, institutions may be more inclined to invest in robotic surgical technology, thereby enhancing the accessibility of advanced methods like the robotic-assisted DIEP flap.
The introduction of the DIEP flap has significantly transformed the options available for women considering breast reconstruction. Although it is considered the “gold standard” for autologous breast reconstruction, the traditional DIEP flap is invasive and may result in lengthy abdominal fascial incisions, which can lead to increased pain, bulging, and hernias. The robotic-assisted DIEP flap marks the next advancement in this field, providing all the advantages of traditional DIEP flap reconstruction while reducing morbidity at the donor site. While the robotic-assisted approach currently involves longer operative times and higher costs, there are numerous potential improvements that could alleviate these disadvantages. The robotic-assisted DIEP flap is a crucial development in enhancing the surgical experience for women undergoing breast reconstruction.
Received date: March 12, 2024
Accepted date: May 09, 2024
Published date: June 03, 2024
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)’ autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2024 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.

Video Following robotic-assisted ligation, the vascular pedicle of the deep inferior epigastric perforator flap is externally extracted and removed from the intra-abdominal cavity.
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
The authors describe various patient and breast-related factors that influence surgical outcomes while also addressing some techniques and principles for aesthetic microsurgical reconstruction.
This systematic review and meta-analysis provide a pragmatic evaluation of drain-free versus drain-based DIEP flap techniques for breast reconstruction, challenging the traditional reliance on drainage. By analyzing postoperative outcomes, the study highlights the potential for refining surgical strategies to enhance patient comfort and recovery without compromising safety. The findings offer a neutral perspective, suggesting that clinical practice may not necessarily depend on the use of drains. This revelation prompts medical professionals to reassess existing surgical approaches and may catalyze a paradigm shift in postoperative care. Presented with clear narrative and rigorous data analysis, the article encourages readers to consider the broader implications of surgical innovations on patient care protocols.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
Division of the lateral plantar artery does not jeopardize the foot circulation because of anastomosis of the lateral plantar artery with the dorsalis pedis artery at the first intermetatarsal space. However, care should be taken with patients with peripheral artery occlusive disease and the flow of dorsalis pedis artery should be confirmed before surgery. Given the advantages of sizable vessel, easy dissection, and proximity to the defect, we believe that the lateral plantar artery might be a valuable option as recipient vessel for lateral plantar forefoot reconstruction.
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
The ALT and AMT flaps are the most commonly used thigh free flaps for intraoral reconstruction. Recently, PAP flap has been proposed as an alternative. This study aimed to compare the thickness of these thigh flaps and to identify the factors influencing flap thickness in our population.
A thin profunda artery perforator flap harvested from the left thigh is shown in this video. Preoperative computed tomographic angiography is used to assess morphology of the perforators and its branches, pedicle length and vertical location of the two branches from the ischial tuberosity. These measurements are translated on to the patient. Locations of both branches are confirmed with a handheld doppler. The authors concluded that preoperative computed tomographic angiography is a useful technique to provide detailed anatomic information on morphology of perforators, course through the septum or muscle above the deep fascia and skin thickness. Computed tomographic angiography allows quick and easy assessment of the whole vascular anatomy of the leg and helps to arrive at the decision about selection of the best flaps based on the characteristics of the defect and on the individual anatomy of the patient.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This systematic review and meta-analysis provide a pragmatic evaluation of drain-free versus drain-based DIEP flap techniques for breast reconstruction, challenging the traditional reliance on drainage. By analyzing postoperative outcomes, the study highlights the potential for refining surgical strategies to enhance patient comfort and recovery without compromising safety. The findings offer a neutral perspective, suggesting that clinical practice may not necessarily depend on the use of drains. This revelation prompts medical professionals to reassess existing surgical approaches and may catalyze a paradigm shift in postoperative care. Presented with clear narrative and rigorous data analysis, the article encourages readers to consider the broader implications of surgical innovations on patient care protocols.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
The authors describe various patient and breast-related factors that influence surgical outcomes while also addressing some techniques and principles for aesthetic microsurgical reconstruction.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
A 43-year-old male presented with a 10-cm tumor in the left forearm that had grown progressively for 5 months. A pathological analysis confirmed a diagnosis of high-grade fibrosarcoma. The image on the rare entity contributes to the notion of this disease.
Dysphagia is an important consequence of cancer treatment and has overarching implications on quality of life. Using the FOIS, we demonstrated that swallowing function may be worse in the long term in patients with OPSCC undergoing triple therapy, although this finding did not reach statistical significance. This study emphasizes the importance of diligent selection in patients undergoing TORS to avoid poor functional swallowing outcomes, particularly in those that may need adjuvant chemoradiation therapy. A study with a larger sample size may determine the significance of these trends.
This study examined two surgical techniques commonly used for turbinate reduction (i.e., submucous resection and partial excision) and their associated complications in functional nasal surgery patients. So far, there has been no direct comparison of these two methods with endpoints of epistaxis and nasal congestion. It was found neither technique was statistically significantly different from the other, so both are clinically useful with a low incidence of postoperative epistaxis.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The article describes the case of a 27-year-old man who suffered from scarring at the penopubic junction as a result of penis lengthening surgery. The authors explain how they repaired the penopubic junction by using a pedicled superficial circumflex iliac artery perforator flap. The authors also propose a safe and effective hybrid technique for harvesting superficial circumflex iliac artery perforator flaps. According to the authors, the pedicled superficial circumflex iliac artery perforator flap is an effective method for reconstructing the penopubic scar.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
The article discusses a complex case of a 51-year-old Chinese woman diagnosed with a pituitary neuroendocrine tumor in the clivus, characterized by its invasive nature and atypical symptoms, leading to diagnostic challenges between chordoma and chondrosarcoma. Achieving a correct diagnosis through a transsphenoidal biopsy enabled effective surgical removal of the tumor without complications. Highlighting the critical role of biopsy for accurate diagnosis, especially with atypical imaging, the study showcases the efficacy of minimally invasive transnasal endoscopic biopsy techniques. It emphasizes the importance of a multidisciplinary approach for optimal patient outcomes in complex pituitary tumors, underlining the need for vigilance and adaptability in managing such rare conditions. This contributes valuable insights to the medical field, particularly for neurosurgery, otorhinolaryngology, and endocrinology practitioners.
This article presents a case study on the successful replantation of a pediatric lower lip after a dog bite, focusing on artery-only microanastomosis. It highlights the challenges and effective strategies in pediatric microvascular surgery, particularly emphasizing the importance of specialized surgical techniques and thorough postoperative care. Alongside the case study, an extensive literature review supports the feasibility of artery-only anastomosis and the traditional yet critical use of leech therapy for managing venous congestion. This research is vital for medical professionals specializing in pediatric surgery, offering key insights into improving both functional and aesthetic outcomes for young patients. Additionally, it identifies gaps in long-term research and stresses the need for ongoing studies to refine treatment protocols, making it an indispensable resource for enhancing patient care and outcomes in pediatric reconstructive surgeries.
This article explores the transformative impact of artificial intelligence on healthcare, providing readers with insights into its role in enhancing diagnostics, treatment, and patient care. It highlights potential cost savings and promotes a proactive approach to health management. The piece underscores advancements in robot-assisted surgery and artificial intelligence-enhanced virtual nursing, alongside efficient data management in healthcare. It addresses challenges and ethical considerations in integrating artificial intelligence, emphasizing the need to maintain clinical skills and empathy. Beneficial for readers, the article advocates a balanced approach that melds technological innovation with foundational healthcare principles, aiding informed decision-making in evolving healthcare landscapes.
The PLOSEA technique detailed in this study addresses the significant challenge of managing large vessel size discrepancies in microvascular surgery with an innovative and accessible method. By partially obliterating the larger vessel lumen before anastomosis, the technique reduces risks of thrombosis and misalignment, simplifying the procedure without sacrificing effectiveness. This advancement is particularly valuable as it allows surgeons with varying levels of experience to perform complex reconstructions with greater confidence and improved patient outcomes. A key feature is the inclusion of a detailed video demonstration, providing a dynamic and comprehensive visual guide that surpasses traditional static images. This video meticulously elucidates each procedural step, enhancing understanding and facilitating the practical application of the technique. Emphasizing technical precision, patient safety, and surgical efficiency, this study offers a compelling narrative for medical professionals. The transformative impact of the PLOSEA technique on surgical practice underscores its importance, presenting a novel approach that can enhance the quality of care and expand the capabilities of microsurgeons worldwide.
Robotic-assisted surgery has established a presence in specialties such as gynecology, urology, and general surgery; however, its integration into plastic surgery has lagged. This review substantially enhances the sparse literature on robotic techniques within the field of plastic surgery. Importantly, it features a comprehensive case study that elucidates the procedural steps executed by the surgical team, thereby rendering these advanced surgical methods more accessible and understandable to plastic surgeons. This enhancement facilitates the broader adoption of next-generation robotic-assisted Deep Inferior Epigastric Perforator (DIEP) flap harvest techniques. The article is timely and possesses significant potential to advance the field of plastic surgery, meriting extensive discussion and scrutiny within the clinical community. Upon addressing reviewers' concerns, this pivotal article could have a profound impact on the realm of breast reconstruction surgery.
ResponseThank you for this recognition of the importance of our manuscript.
This article represents a substantial advancement in the field of breast reconstruction by exploring the application of robotic-assisted surgery in the harvesting of Deep Inferior Epigastric Perforator (DIEP) flaps. It effectively demonstrates how integrating robotic technology can enhance the efficiency of flap harvesting processes, potentially reducing operative times, minimizing morbidity, and improving both aesthetic and functional outcomes. The article provides detailed descriptions of the procedures, as well as preoperative and postoperative considerations, forming a thorough guide that could significantly shape future surgical techniques. Additionally, the included case study offers practical insights into the challenges and solutions associated with implementing robotic systems in complex reconstructive surgeries. This innovative approach, combined with an in-depth analysis, positions the article as a significant contribution to the existing literature on robotic surgical applications, establishing a new standard for excellence in breast reconstruction and encouraging further research. Nonetheless, certain issues must be addressed to further solidify the findings and implications of this study.
The review article begins with an overview of recent advancements in breast reconstruction, particularly highlighting the application of robotic assistance in harvesting Deep Inferior Epigastric Perforator (DIEP) flaps. It presents a detailed analysis of the associated surgical procedures, augmented by a case study that demonstrates the practical implementation of this technology in reconstructive surgery. However, the research exhibits significant limitations, including the lack of standardized preoperative evaluations and ambiguous criteria for participant selection, which undermine its impact. Furthermore, the article does not address nerve preservation or conduct a comprehensive cost-benefit analysis of robotic versus traditional surgical methods—essential components for enhancing its clinical relevance and scholarly value. It is advisable for the authors to address these shortcomings to improve the manuscript’s suitability for publication.
Aiello C, Choe J, Suri K, Smith ML, Selber JC, Sugiyama G, Tanna N. Advancing breast reconstruction: Next-generation robotic-assisted deep inferior epigastric perforator flap techniques. Int Microsurg J 2024;8(1):3. https://doi.org/10.24983/scitemed.imj.2024.00185