The etiopathogenesis of laryngopharyngeal reflux (LPR) involves direct caustic irritation of gastroduodenal materials and indirect laryngeal reflexes invoked by refluxate. The most common symptoms include throat clearing, persistent cough, globus pharyngeus, and hoarseness. There is only a weak correlation between LPR symptoms and endoscopic findings. It is not recommended to make a diagnosis of LPR solely based on laryngoscopic results. LPR treatment generally requires an aggressive approach, including high doses of proton pump inhibitors over long periods (twice daily for 3-4 months). Additional management options include lifestyle changes, dietary modifications, weight reduction, and exercise. Laparoscopic anti-reflux surgery has been shown to reduce LPR-associated symptoms; however, surgery should only be considered for patients who have a high quantity of refluxate and esophageal complications. In this review article, we suggest a multidisciplinary approach to LPR diagnosis, involving otolaryngologists, gastroenterologists, and pulmonologists. Based on the latest findings, we propose an algorithm to facilitate the assessment and management of LPR.
Laryngopharyngeal reflux (LPR), also referred to as extra-esophageal reflux, supra-esophageal reflux, or silent reflux, refers to a condition in which gastroduodenal content rises up the esophagus and affects the throat, specifically the laryngopharynx [1-6]. In some cases, gastric content may even reach the nasal cavities and/or ears via the Eustachian tubes, which can exacerbate rhinitis, sinusitis, or otitis media [7-9].
Otolaryngologists and gastroenterologists differ in their definitions and management of LPR [4,10-12]. Otolaryngologists treat LPR as a relatively new clinical entity, whereas gastroenterologists treat LPR as a rare extra-esophageal manifestation of gastroesophageal reflux disease (GERD) [10,13]. Gastroenterologists have questioned whether reflux contributes to LPR-related symptoms in patients with no GERD-associated manifestations [11]. Otolaryngologists have pointed out that LPR is a multifactorial syndrome that also involves gaseous and/or nonacid refluxate [14,15].
In this article, we examine the clinical manifestations, diagnosis, and current recommended treatments of LPR. Based on the latest findings in LPR research, we propose an algorithm aimed at facilitating the assessment and management of LPR.
Despite similarities between LPR and GERD, these are two distinct disease entities. The retrograde flow of gastroduodenal contents into the esophagus and/or adjacent structures can lead to complications or troublesome reflux-associated symptoms, such as throat clearing, heartburn, and globus pharyngeus. Reflux diseases can be categorized as LPR, erosive esophagitis, and nonerosive reflux disease (NERD). Cases of erosive esophagitis and NERD are categorized as GERD [16].
In GERD, the reflux of gastric contents is limited to the esophagus. In LPR, the reflux of gastric content affects the larynx and pharynx [12]. Despite occasional cross-diagnoses of GERD and LPR, there are essential differences (Table 1). GERD is accompanied by acidity and heartburn (retrosternal burning), which is rarely encountered in LPR patients [12]. In GERD, reflux and acidity typically occur during the night (nocturnal refluxers). In LPR, reflux typically occurs during the day (daytime refluxers) [12]. LPR symptoms occur when patients are in an upright position during periods of physical exertion (e.g., bending over, Valsalva, and exercise) [11,12,17], whereas GERD reflux occurs while patients are lying down.
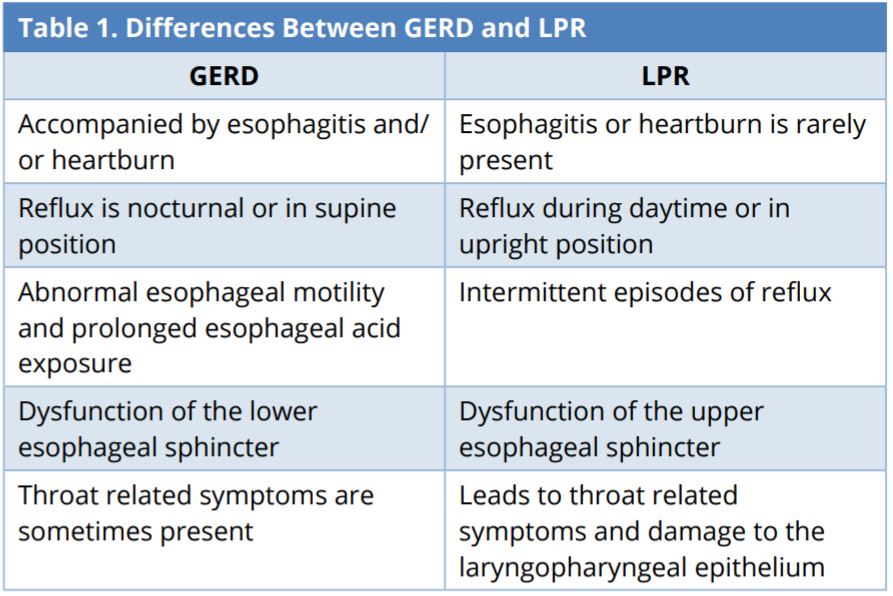
GERD, gastroesophageal reflux disease; LPR, laryngopharyngeal reflux.
Sphincters that prevent the stomach reflux from moving backwards play an important role in both diseases. Many GERD patients experience dysmotility and prolonged acidity when the lower esophageal sphincter malfunctions, thereby allowing stomach acid to move up the esophagus, causing heartburn [12,18]. LPR is associated with a failure of the upper sphincters, which allows the acid to move up to the throat and even into the nose or middle ears [7-9,12]. LPR patients commonly present tissue damage of the laryngopharyngeal epithelium [12,19].
The typical manifestations of GERD include heartburn, regurgitation, and chest pain. The typical manifestations of LPR include asthma, chronic cough, hoarseness, globus sensation, and laryngitis in adult patients. LPR patients do not usually report symptoms of heartburn, which is common in GERD patients. It is often difficult to differentiate between LPR and GERD due to overlap in the symptoms. LPR rarely occurs in isolation; i.e., without concomitant symptoms typical of GERD [20,21]. Researchers have identified a correlation between the presence of LPR and the severity of GERD; however, similarities between the two may lead to underestimates of the incidence of LPR [22]. The diagnostic sine qua non of GERD, namely endoscopic esophageal mucosal breaks (erosion or ulceration), has been reported in only 25% of patients with LPR [12,18].
The etiopathogenesis of LPR involves direct as well as indirect mechanisms. The reflux components, which contain hydrochloric acid, pepsin, and bile acids, can irritate the laryngeal mucosa [2,23-26]. Reflux episodes in the esophagus can occur up to fifty times without harmful effects, whereas reflux in the larynx cause mucosal damage after just three episodes [13,23]. Direct refluxate irritation can cause local mucosal inflammation and subsequent laryngospasm. Up-regulated sensitivity in laryngeal sensory endings can lead to coughing and choking [27].
The indirect mechanism involves laryngeal reflexes evoked by refluxate that does not reach laryngeal tissue. Reflex evokes a vagally mediated change, resulting in clinical symptoms, including chronic cough and asthma-like symptoms though bronchoconstriction. A decrease in the resting tone of the upper and lower esophageal sphincters and increases in intraabdominal pressure are also associated with the refluxate bolus and subsequent occurrence of LPR [11,28-35].
Direct and indirect irritation can have consequences for the vocal cords, such as vocal edema, pseudosulcus of vocal cords, contact ulcers, and contact granulomas associated with hoarseness, globus pharyngeus, and sore throat [14,18]. Pseudosulcus of vocal cords associated with infraglottic edema has been identified in 90% of patients with LPR [14,36].
Eating habits, tight clothing, stress, and excess weight have also been shown to contribute to LPR. This condition is more common among people who habitually consume acidic, oily, or spicy preparations. The consumption of alcohol is also a contributory factor. Tight clothing sometimes causes acid to swell up into the food pipe resulting in LPR. Stress can induce an increase in acidity levels and has been shown to cause LPR. Overweight people are more prone to this condition [2].
The most common symptoms of LPR include throat clearing, persistent cough, globus pharyngeus, and hoarseness [3,11,14,18,37]. Globus pharyngeus is a non-painful sensation of a lump or foreign body in the throat [38]. Heartburn is the most common symptom of GERD, occurring in more than 75% of cases [39]; however, fewer than 40% of patients with LPR report heartburn [11,12]. Reichel and Issing reported that Barrett’s metaplasia or grade B esophagitis was diagnosed only in patients in which heartburn was the main presenting symptom [40]. This suggests that upper gastrointestinal endoscopy (UGE) may be indicated in LPR patients reporting heartburn as their main complaint in order to exclude structural injuries or neoplasms.
Many of the LPR symptoms are nonspecifically associated with nasal conditions, such as allergies and postnasal drip [41,42]. LPR has been shown to have a negative effect on nasal resistance and nasal congestion [43]. Treatment associated with LPR may improve subjective and objective nasal problems [43]. Studies also revealed an association between LPR and halitosis, taste, or smelling disorders [44,45]. Researchers have recently reported that acid reflux may be associated with middle and inner ear problems, such as otitis media, tinnitus, and peripheral vertigo [46-48]. The mechanism underlying these inner ear disorders may be associated with reflux material (specifically hydrochloric acid and pepsin) leaking into the middle ear via the Eustachian tubes and affecting osseous structures.
Reflux Symptom Index (Table 2)
The reflux symptom index (RSI) can assist in diagnosing LPR [49,50]. The RSI is derived using a simple nine-item questionnaire in which patients rate the severity of their LPR symptoms on a Likert scale, with 0 representing no problem and 5 representing extreme problems. The maximum score is 45, and a score of more than 13 is diagnosed as abnormal acid reflux [50].
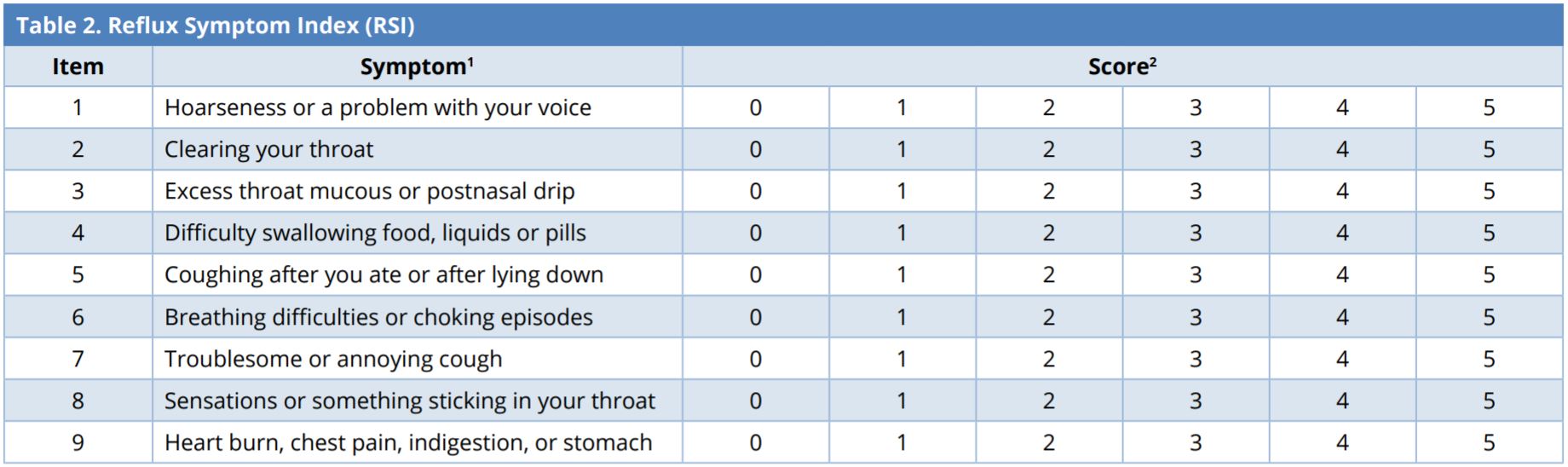
1 Patients are asked to determine how the associated problems affect them within the last month.
2 0-5 rating scale with 0 = no problem and 5 = severe.
Laryngopharyngeal reflux is considered if RSI > 13.
Laryngoscopic Examinations
Laryngoscopic examinations for signs of laryngeal irritation associated with reflux are performed using flexible transnasal or rigid transoral laryngoscopes. One prospective study reported that signs of laryngeal irritation are more often detected with flexible than with rigid laryngoscopes [51]. The key features of laryngeal irritation include ventricular obliteration, vocal fold edema, subglottic edema (pseudosulcus) as well as thickening, redness, and edema mainly localized in the posterior larynx involving posterior pharyngeal wall, arytenoids, and interarytenoid area [6,36,52].
Nonetheless, there is only a weak correlation between LPR symptoms and endoscopic findings. In a prospective study that included 52 nonsmokers, laryngoscopy revealed signs of laryngeal irritation in over 80 % of cases [51]. Furthermore, the laryngoscopic diagnosis of LPR can be highly subjective, depending largely on the expertise and experience of the clinician [53]. It is notable that the laryngeal irritation signs may also be the result of non-reflux etiologies, such as allergy, smoking, or voice abuse [21]. Accurate laryngoscopic assessment of LPR is likely to be difficult, and it is not recommended to make a diagnosis of LPR solely based on laryngoscopic results [21,37,42,53].
Reflux Finding Score (Table 3)
The RFS is an eight-item measure used by clinicians to rate the severity of signs of inflammation revealed in laryngoscopic examinations, including subglottic edema (pseudosulcus), ventricular obliteration, erythema or hyperemia, vocal fold edema, diffuse laryngeal edema, posterior commissure hypertrophy, granuloma or granulation tissue (Figure 1 and Video 1), and thick endolaryngeal mucus. The clinicians rates the severity of each symptom by assigning scores from 0 (normal) to 26 (worst possible score). LPR can be diagnosed with 95% certainty in cases where the RFS exceeds 7 [54]. It can also be used to track treatment responses in patients. The RFS and RSI both help to improve the accuracy of LPR diagnoses and evaluate the efficacy of treatments. The RFS is a cost-efficient method, which can be included in otolaryngologic examinations to facilitate the diagnosis of LPR [49,50,54].
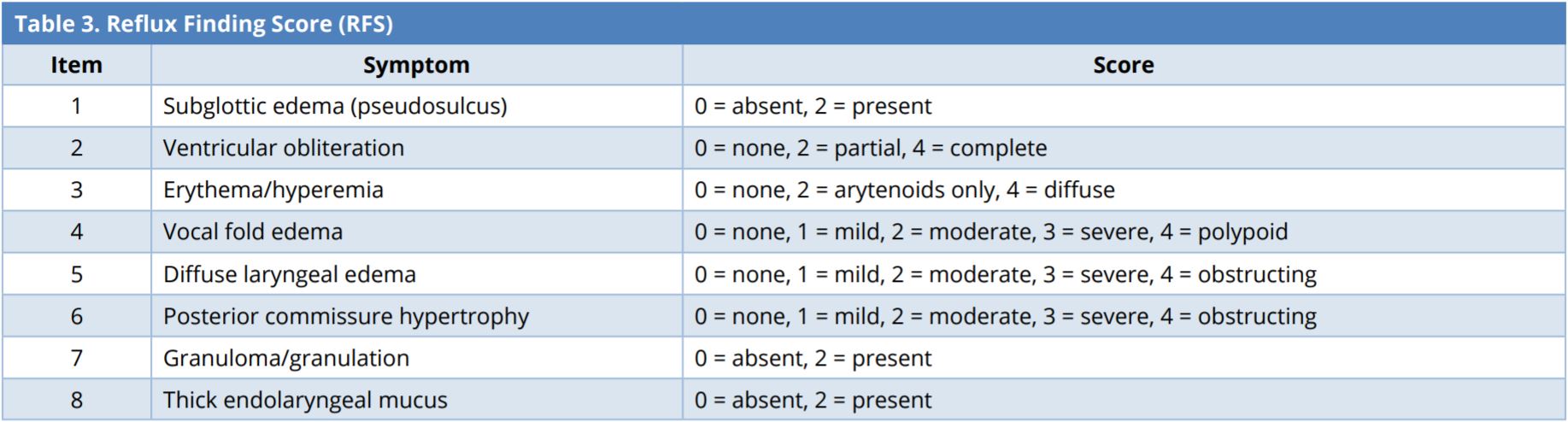
Laryngopharyngeal reflux is considered if RFS > 7.
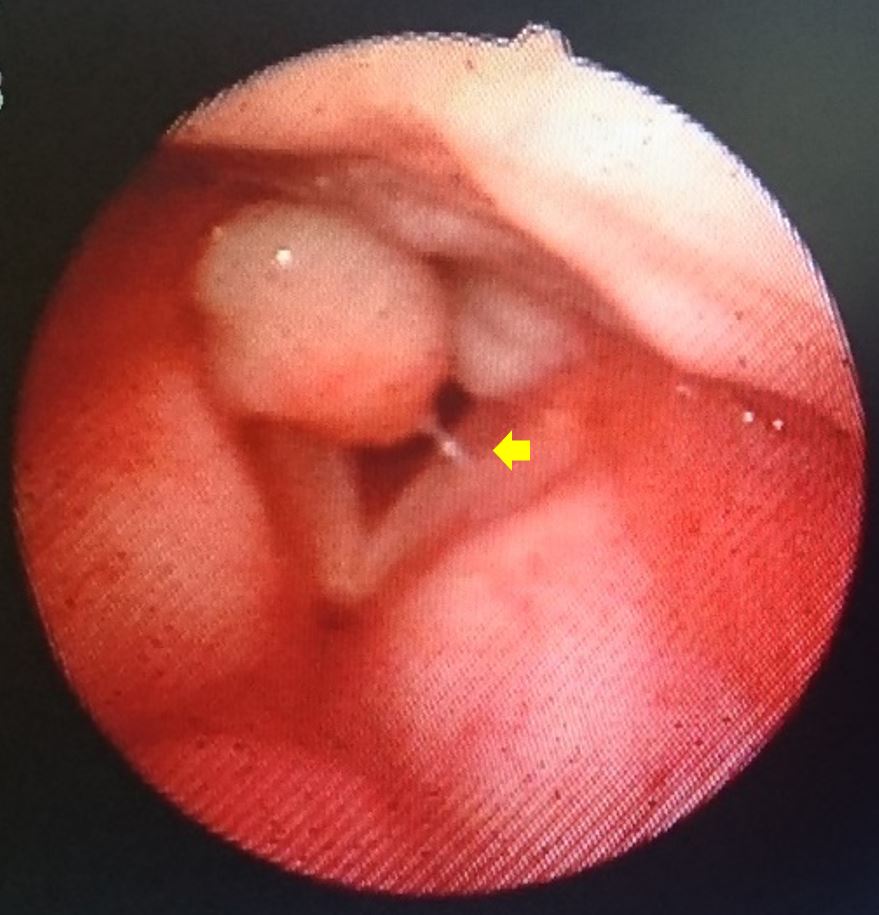
Figure 1. Laryngoscopic image showing a larynx with large bilateral granulomas on the surface of arytenoids. A prominent pseudosulcus is identified by the arrow.
Video 1. Laryngoscopic examination for signs of laryngeal irritation associated with reflux. Large bilateral granulomas are identified on the surface of arytenoids.
Dual-Sensor pH Probe
The 24-hour dual-sensor pH probe (simultaneous esophageal and pharyngeal) is considered the gold standard in the diagnosis of GERD, with sensitivity of 93.3% and specificity of 90.4%, when using a cut-off value of 4.5% of total time with pH < 4 during a 24-hour period [55]. Ambulatory pH probe-monitoring is often applied to evaluate the efficacy of drug treatment in cases of LPR [12]; however, it is considered a less reliable test for confirming LPR [11,14]. This is primarily due to the difficulties involved in interpreting pH monitoring data and a lack of consensus on normal pH limits, number of events, and probe placement [11,56,57]. Another concern with pH probe-monitoring is its inability to detect gaseous and/or nonacid refluxate, which are potentially harmful to the laryngopharynx. As a result, intraluminal impedance testing is generally regarded as a superior approach [14].
Upper Gastrointestinal Endoscopy
UGE is also referred to as esophagogastroduodenoscopy (EGD). UGE can detect signs associated with GERD, such as mucosal injury, esophagitis, and Barrett esophagus as well as other complications and malignancies; however, UGE has proven less useful in detecting LPR than in identifying GERD [12,18]. In one study, UGE revealed esophageal lesions in 50% of GERD patients and in less than 20% of LPR laryngitis patients [58]. For patients presenting warning signs of complications (i.e., chronic cough, hoarseness, or dysphagia) or malignancies, it is recommended that they be referred to specialists, such as otolaryngologists, gastroenterologists, and pulmonologists [11].
Other Tests
Other tests have been also used to facilitate the diagnosis of LPR. Barium swallow esophagrams allow clinicians to screen the esophagus for related pathologies [12]. Alternative diagnostic techniques have also been devised to explore the associations between the LPR and histomolecular findings, including salivary epidermal growth factor, immunologic markers, laryngeal mucosa gene expression, and histologic changes [59-61].
It is important that LPR is diagnosed and treated effectively. A failure to do so can lead to chronic cough, granulation of arytenoids, and/or ulcers on the vocal folds. This condition has also been linked to asthma, bronchitis, chronic rhinitis, sinusitis, and otitis media. Researchers have reported potential associations between acid reflux and esophageal, oropharyngeal, and hypopharyngeal neoplasms [62].
Studies have found that approximately half of the patients with mild LPR can avoid symptoms by implementing changes in their lifestyle [27]. These lifestyle changes are related to eating, drinking, and other habits. For example, raising the bed at the head side has been shown to prevent LPR symptoms. It is also recommended that patients quit smoking, lose weight, and wear loose clothing.
Dietary Modifications
LPR symptoms may be reduced by changing dietary habits. Patients are advised to eat food early (at least two hours before bedtime) to allow time for digestion before lying down [11]. Moreover, patients with acid reflux diseases are also advised against ingesting too much coffee [26] or carbonated drinks, which are known to affect acidity and cause reflux [63].
Spicy foods irritate the lower esophageal mucosa leading to heartburn and a burning sensation in their chests. High-fat foods and chocolate are known to prolong gastric emptying, and high-fat foods take longer to digest and have been associated with higher incidences of GERD and erosive esophagitis [64]. Note that some studies have reported that a high-fat diet has no effect on esophageal acid exposure or transient relaxation of lower esophageal sphincter [65-67]. Nonetheless, patients should be advised to avoid fatty diets to facilitate digestion and promote overall health. A high-calorie diet can also affect esophageal acid exposure. One study found that a high-calorie diet was associated with prolonged acidity in the stomach, which could aggravate reflux symptoms [68].
Lifestyle Changes
Reflux diseases are known as lifestyle diseases. Patients are therefore advised to avoid smoking, as it is known to cause acid production [11]. Smoking cigarettes is directly correlated with acid retention leading further to slow clearance of esophageal acid. Smokers have also been shown to have a higher incidence of reflux symptoms, compared to non-smokers [69,70].
Researchers have identified a direct link between the consumption of alcoholic drinks and acid exposure and reflux. Alcoholic drinks of all types are a direct cause of heartburn. Consuming large quantities of alcohol poses the same risks, regardless of whether it was beer, wine, or spirits. Wine and beer have also been found to cause reflux, even in small quantities. Endoscopic studies have revealed that white wine and beer have similar effects on reflux esophagitis and abnormal pH levels. White wine has a more pronounced effect on acid exposure than does red wine [71-74].
Weight Reduction
Weight reduction is essential for patients suffering from LPR and GERD, due to the prevalence of these symptoms in obese patients. Researchers have shown a strong relationship between obesity and acid reflux [75], and a high body mass index (BMI) is directly related to acid reflux [76,77]. Weight gain can aggravate reflux symptoms and weight loss can have the reverse effect, allowing patients to reduce taking reflux medications [78,79].
Exercise
Patients are advised to participate in exercise sessions of at least 30 minutes each day as a guard against reflux symptoms. Patients who are less active physically are more at risk of developing reflux problems [80,81].
Acid suppression via proton pump inhibitors (PPIs) is the mainstay of medical treatment for LPR. H2-receptor antagonists (H2AT), prokinetic agents, and mucosal cytoprotectants (e.g., sucralfate) may provide additional benefits [11,14,82]. Neuromodulators (e.g., tricyclic antidepressants, gabapentin, and pregabalin) may be an option for patients with symptoms that are not relieved by acid suppression, particularly in cases where laryngeal sensitivity (neuropathy) appears to be contributing to symptoms of LPR [11,33,35,83]. LPR treatment generally requires an aggressive approach, including high doses of PPIs over long periods (twice daily for 3-4 months) [10,12,14]. Note that the efficacy of PPIs in LPR treatment is controversial and has not been conclusively proven [84-88].
Surgery is usually a last resort in LPR treatment [11,89-91]. Patients should be warned that the response of their laryngeal symptoms to surgery is uncertain. Surgery should only be used in cases where patients responded to PPI therapy but did not achieve complete relief of LPR symptoms.
There are, as yet, no multidisciplinary approaches to the assessment and management of LPR [13]. It is still difficult to differentiate among reflux disorders, such as LPR and GERD, due to an overlap of symptoms. There is also some controversy about the routine use of endoscopy for patients with reflux disorder on their initial visit. Based on previous research, we developed the algorithm shown in Figure 2, with the aim of streamlining the assessment and management of reflux disorders, including LPR and GERD.
Patients with symptoms suggestive of complications or malignancies (e.g., dysphagia) require a referral to specialists for an endoscopic examination. Chest pain is seldom a symptom indicative of LPR; therefore, it is important to differentiate between cardiac from non-cardiac chest pains before considering LPR as a potential culprit. Chest X-rays may be required to exclude the possibility of lung disorders for patients presenting with chronic cough (2 or more weeks). Hoarseness is sometimes associated with uncomplicated LPR; however, lingering hoarseness for more than 2 weeks warrants an investigation of potential complications, including underlying vocal cord paralysis or lesions. This would involve referral to an otolaryngologist for laryngoscopic examination.
In primary care units, the diagnosis may be based primarily on LPR-associated symptoms and a therapeutic trial that includes lifestyle changes, dietary modifications, and the short-term use of PPIs. If LPR-related symptoms can be resolved within 2-4 weeks of a therapeutic trial using PPIs or H2AT, titrating therapy at the lowest dosage may be required for 2-3 months. Patients who show a less than complete improvement in symptoms may require a maintenance regimen for 2-3 months before initiating titrating pharmacologic treatment. It is suggested that patients are referred to specialists in the event that short-term therapeutic trials fail.
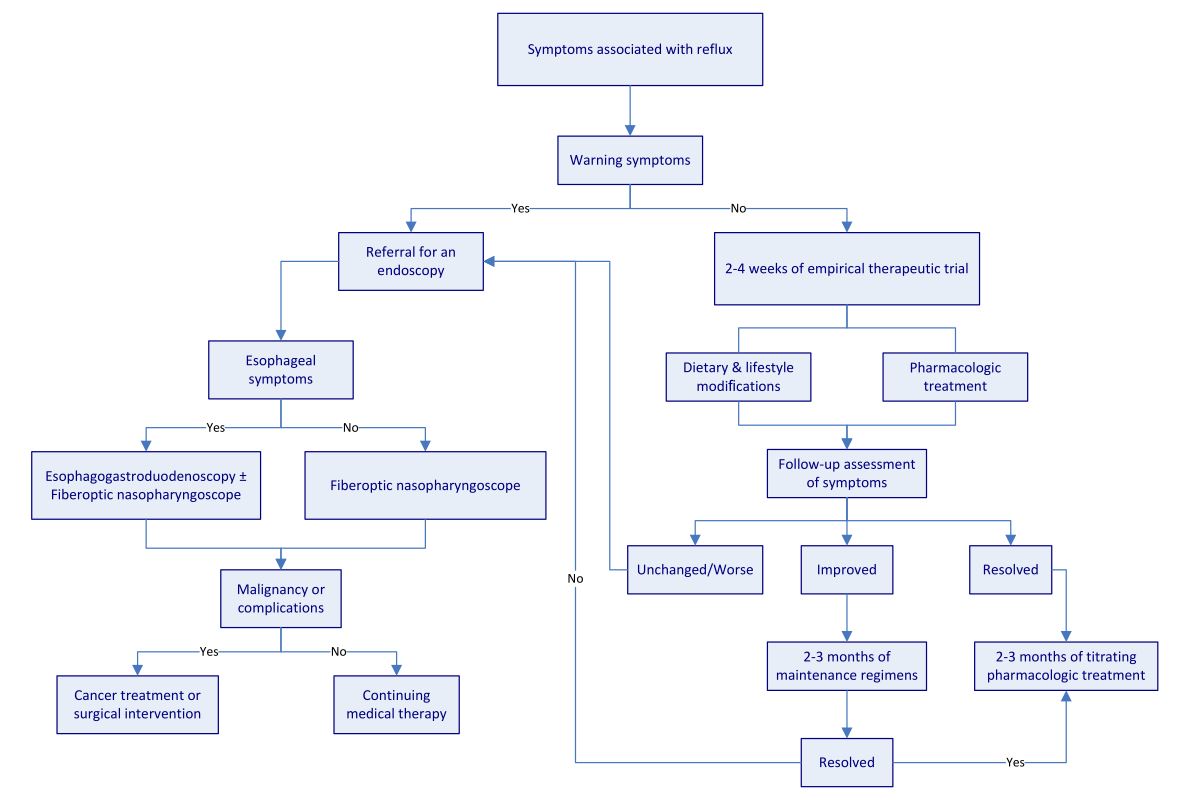
Figure 2. Algorithm for assessment and management of reflux disease.
LPR is clinically distinct from GERD. LPR is very common, particularly among the elderly. Numerous existing methods provide useful diagnostic information on LPR, including endoscopic evidence of mucosal damage, demonstration of reflux events by multichannel impedance and pH-monitoring studies, radiography, esophageal manometry, spectrophotometric measurement of bile reflux, and mucosal biopsy. Nonetheless, there remains some controversy regarding the appropriate course of action in the diagnosis of LPR, and no test is considered conclusively reliable.
LPR symptoms can be alleviated or eliminated by adopting changes in lifestyle, such as dietary, behavioral, and lifestyle habits. Patients are also advised to avoid sweet and fried foods, refrain from smoking and drinking, and wear loose comfortable clothing. They should also try to reduce stress in their lives and reduce their weight. Further investigations into alternative causes of laryngeal symptoms, including allergy, sinusitis, or pulmonary disorders, should be considered for patients who fail to respond to LPR treatments.
The risk of misdiagnosis based on reliable medical history records is relatively small. When the diagnosis is in question or the therapeutic response to PPIs is unsatisfactory, referral to a specialist is required to confirm the diagnosis of LPR.
Received date: March 01, 2018
Accepted date: May 30, 2018
Published date: January 09, 2019
This study was sponsored by grants from the Medical Affairs Bureau, Ministry of National Defense (MAB-107-099), and Taoyuan Armed Forces General Hospital (AFTYGH No. 10734).
None
© 2019 The Author. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Video 1. Laryngoscopic examination for signs of laryngeal irritation associated with reflux. Large bilateral granulomas are identified on the surface of arytenoids.
This investigation delineates a pivotal association between socioeconomic inequities, quantified via the Area Deprivation Index (ADI), and an elevated incidence of button battery ingestion in pediatric populations, highlighting a profound public health issue. The results indicate that children residing in socioeconomically disadvantaged areas are at an increased risk of sustaining severe injuries from the ingestion of button batteries, which could lead to elevated morbidity and mortality rates. The study urgently calls for immediate diagnostic and therapeutic interventions to avert critical health complications and delineates the complex pathophysiology underlying button battery injuries. For clinicians and healthcare practitioners, particularly those within pediatrics and emergency medicine, this manuscript is indispensable. It provides deep insights into the ramifications of socioeconomic disparities on health outcomes, fosters the refinement of diagnostic and therapeutic modalities, and champions preventive initiatives. The authors advocate for intensified parental awareness, the redesign of battery products to enhance safety, and the formulation of healthcare policies that promote equity, aiming to curtail this escalating health challenge.
This article presents a comprehensive discussion of advanced techniques in managing pediatric airway obstructions caused by vallecular cysts. By employing awake fiberoptic intubation and transoral CO₂ laser microsurgery, the authors highlight a thoughtful, evidence-based approach that emphasizes both safety and precision. While not groundbreaking, the depth of analysis in the decision-making process and procedural techniques offers invaluable insights for clinicians, particularly in pediatric otolaryngology. The article serves as a critical reference for handling complex airway cases, balancing innovative practices with established methods. Its significance lies in its contribution to optimizing patient safety, particularly in high-risk infant cases, making it essential reading for healthcare providers dealing with airway management challenges.
Establishing a relationship between a benign disorder and a malignant disease has a certain influence on clinical practice. Clinicians need to remain vigilant with patients with acid reflux disorders and rule out the possibility and presence of head and neck cancer.
Epistaxis (i.e., nosebleed) is a common otolaryngologic emergency; however, it is seldom life-threatening and most minor nosebleeds stop on their own or under primary care from medical staff. Nonetheless, cases of recurrent epistaxis should be checked by an otolaryngologist, and severe nosebleeds should be referred to the emergency department to avoid adverse consequences, including hypovolemic shock or death. This paper reviews current advances in our understanding of epistaxis as well as updated treatment algorithms to assist clinicians in optimizing outcomes.