Introduction: Lymphaticovenular anastomosis (LVA) and vascularized lymph node transfer (VLNT) are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. Vascularized lymph vessel transfer (VLVT) is free of these limitations. We described our experience of a new VLVT technique.
Patients and Methods: Patients with fluid-predominant lymphedema who failed lymphedema therapy were offered surgical intervention. Those with early injury seen on indocyanine green (ICG) lymphography were treated with LVA. Superficial iliac artery perforator (SCIP)-based lymph vessel transfer was offered to those with advanced injury. After lymphographic mapping of the lymph vessels and doppler perforator mapping, thin SCIP flap was harvested. Only the superficial fat layer was included to recruit the lymph vessels while preserving the lymph nodes. The flaps were vascularized with end-to-side or perforator-to-perforator anastomosis.
Results: The SCIP-based lymph vessel flap was performed in 6 patients with extremity lymphedema. Four had upper and 2 had lower extremity disease. One had partial (< 5%) flap loss which was managed with local wound care. All others had uneventful postoperative course. Follow-up was 13-27 months. All experienced prompt relief of symptoms and circumference reduction. At 1 year out, all demonstrated durable symptomatic improvement with correlating improvement on ICG lymphography. Three of 6 patients achieved minimal compression garment use. None developed donor site lymphedema.
Conclusions: Our early experience of the SCIP-based VLVT showed promising result in treating extremity lymphedema and suggested it as a viable alternative treatment to LVA and VLNT.
Video Abstract
Vascularized lymph node transfer (VLNT) and lymphaticovenular anastomosis (LVA) are established microsurgical and supermicrosurgical treatments of extremity lymphedema and are increasingly performed worldwide [1,2]. By harvesting healthy lymph nodes from another part of the body and re-vascularizing them at the affected limb, VLNT is thought to directly drain the edematous limb via "pump” mechanism [3-5] and lymphangiogenesis [3,6,7]. Since its inception in the 80s, groin VLNT based on superficial circumflex iliac vessels had been performed around the world with consistent success [3,8-11]. No serious donor site complications were reported until 2013, when donor leg lymphedema following groin lymph node harvest was initially reported [12,13]. The concern of donor site lymphedema prompted the subsequent developments of submental [14-16], supraclavicular [2,17,18], lateral thoracic [7,19-22], and most recently, omental [23-25] and jejunal mesenteric [26] lymph node transfers. LVA is free of the risk of donor site lymphedema, but it is only effective in patients with early disease.
Vascularized lymph vessel transfer (VLVT) represented a conceptual departure from the accepted belief that transfer of nodal tissue is required for lymphedema restoration [27]. Based on the first dorsal metatarsal vessels, Koshima et al. proposed the original VLVT for patients with advanced lymphedema and reported favorable outcomes [27]. Similar to VLNT, VLVT is thought to mediate its therapeutic efficacy through the peristaltic, pumping actions of healthy lymphatic vessels [4]. We propose a new VLVT technique based on a different donor site and describe our early experience.
Surgical interventions were offered to patients who failed complex decongestive therapy (CDT) as determined by our certified lymphedema therapists. Physiologic procedures including LVA and VLVT were offered to patients with fluid-predominant disease. Fluid-predominant state was determined by the presence of prominent pitting on physical examination, quantitative bioimpedance spectroscopy showing significant limb fluid excess, and magnetic resonance imaging showing minimal lipodystrophy and subcutaneous fibrosis. The selection between LVA and VLVT was based on the severity of lymphatic injury seen on indocyanine green (ICG) lymphographic findings. Patients with preserved, functioning lymph vessels characterized by "linear" lymphographic pattern were offered LVA. Conversely, complete absence of "linear" pattern and/or the presence of "diffuse" pattern suggestive of severe lymphatic injury indicated VLVT. Therefore, the inclusion criteria are fluid-predominant disease and advanced lymphatic injury. The exclusion criteria are solid-predominant disease and early lymphatic injury treatable with LVA.
Surgical Technique
Groin superficial lymph vessels were delineated with ICG lymphography by placing four 0.05 ml intradermal injections of 0.25% ICG solution lateral to the anterior superior iliac spine. After identifying the locations of perforators with Doppler ultrasound, a 10 x 6 cm superficial circumflex iliac artery perforator (SCIP) flap was designed over the perforators to recruit the mapped lymph vessels (Figure 1). The inferior skin incision was made and deepened to include 3-5 mm subcutaneous fat. Cephalad-directed, lateral-to-medial dissection was performed to identify the superficial and deep branch perforators of the superficial circumflex iliac artery (SCIA). The dissection plane was maintained immediately superficial to Scarpa’s fascia to exclude lymph nodes from the flap (Figure 2). In obese patients, the dissection proceeded along a non-anatomic plane superficial to the superficial fascia to keep the flap thin (Figure 2, 3). Upon confirming perforator entry into the flap, the superior skin incision was committed. Retrograde pedicle dissection was performed until adequate pedicle length was achieved. The presence of lymph vessels in the flap was confirmed by scanning the adiposal surface of the flap with Spy ELITE (Kalamazoo, Michigan) (Figure 4). The VLVT flap was transferred to the wrist using radial artery as the recipient vessel for arm lymphedema and to the ankle using posterior tibial artery as the recipient vessel for leg disease. Perforator-to-perforator anastomosis was preferentially performed when feasible. For vessels 0.5 to 0.8 mm, 11-0 nylon was used for anastomosis. For those smaller than 0.5 mm, 12-0 nylon was used. In the absence of recipient perforator, end-to-side anastomosis was required due to size mismatch between the flap arterial pedicle and the recipient artery. Only vascular anastomoses and no lymphatico-venular or lymphatico-lymphatical anastomosis were performed. Full-thickness skin excision with dimension identical to the VLVT flap was performed at the recipient site to facilitate flap inset (Figure 5). The groin donor wound was closed primarily.
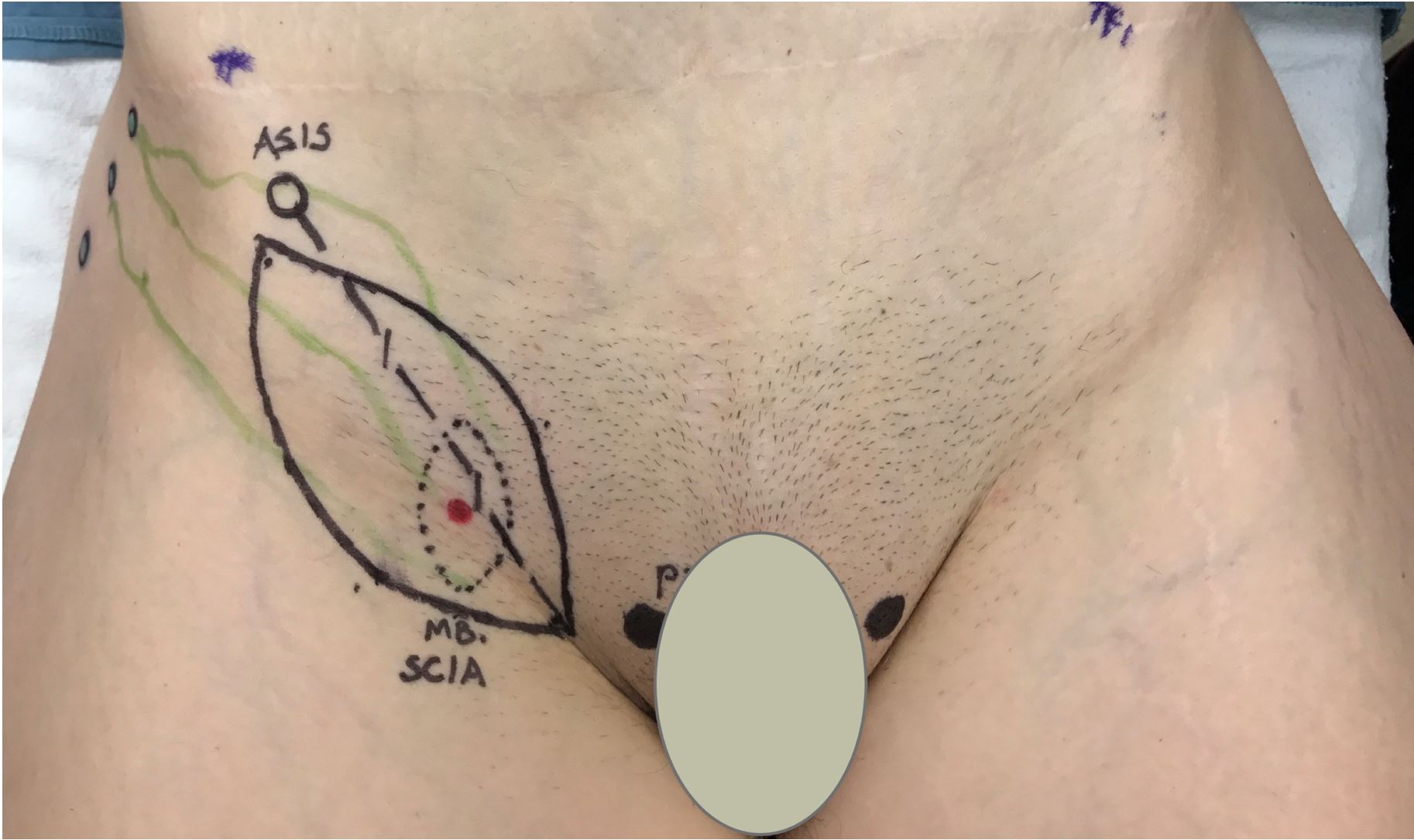
Figure 1. Groin lymph vessels were mapped with ICG lymphography with intradermal injection of 0.25% ICG lateral to ASIS. A thin SCIP-based VLVT flap was designed by identifying SCIA perforators along a line extending from groin crease to ASIS. The width was designed with pinch test to ensure subsequent primary closure of the donor wound. ASIS, anterior superior iliac spine; ICG, indocyanine green; SCIA, superficial circumflex iliac artery; SCIP, superficial iliac artery perforator; VLVT, vascularized lymph vessel transfer.
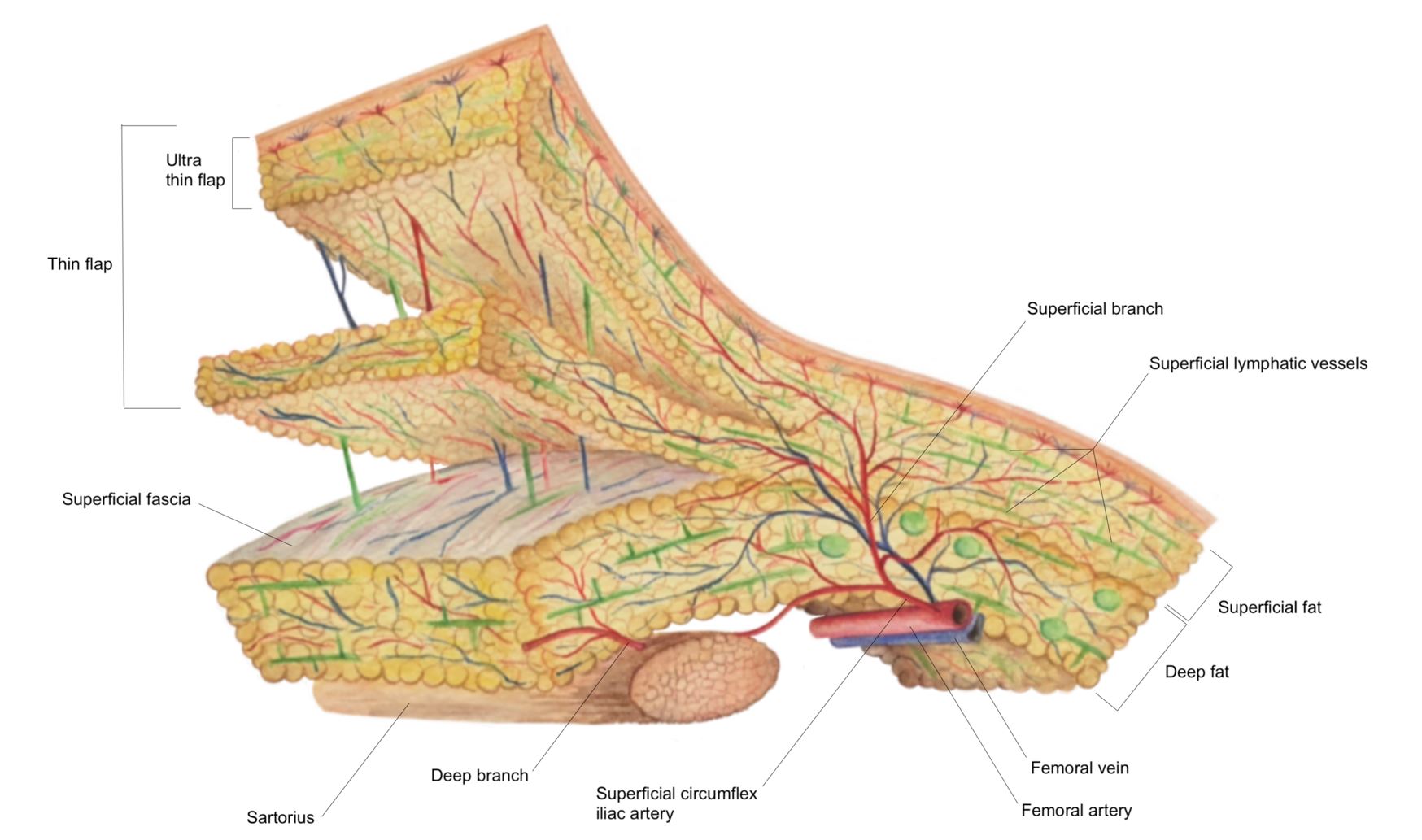
Figure 2. The SCIP-based VLVT flap was typically harvested immediately superficial to the Scarpa’s fascia to preserve the lymph nodes that are anatomically situated deep to the Scarpa’s fascia. This creates a thin flap that facilitates flap inset. In obese patients, the above technique would produce a thick flap. In these patients, we harvested the flap along a non-anatomic, artificially created plane created at 3-5 mm under the dermis. SCIP, superficial iliac artery perforator; VLVT, vascularized lymph vessel transfer.
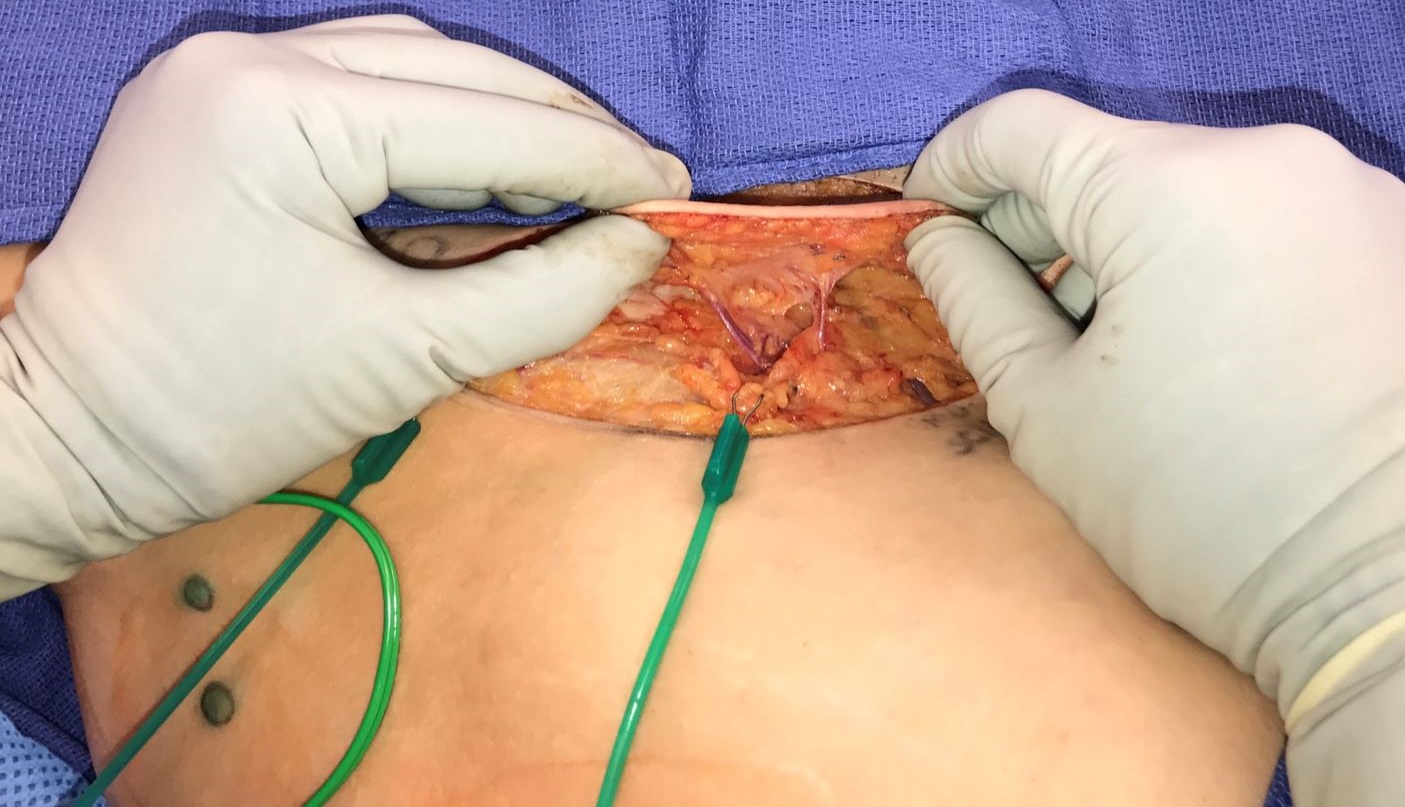
Figure 3. Profile view of a thin SCIP-based VLVT flap. By maintaining a 3-5 mm thickness during flap elevation, the resulting flap would include subdermal lymphatic vessels while minimizing flap bulk. This facilitated flap inset and prevented contour deformity frequently seen in lymph node transfer. Superficial circumflex iliac artery superficial branch perforator (right) and superficial circumflex iliac vein (left) could be seen. SCIP, superficial iliac artery perforator; VLVT, vascularized lymph vessel transfer.
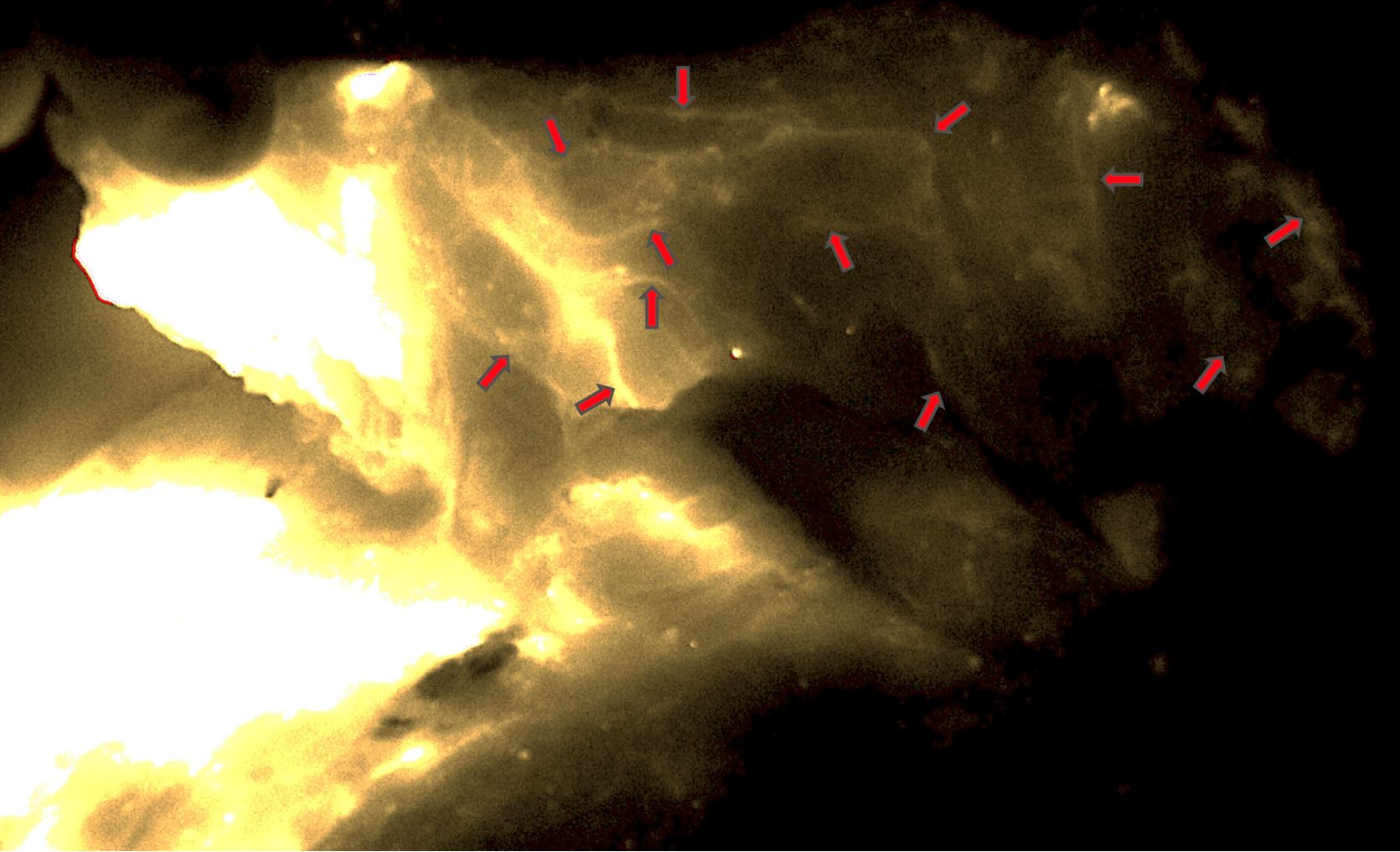
Figure 4. Dense lymphatic network seen in the VLVT flap (red arrows). The image was taken from the adiposal side of the flap. The bright areas on the left side represented the ICG injection site. No lymph nodes were seen in the flap. ICG, indocyanine green; VLVT, vascularized lymph vessel transfer.
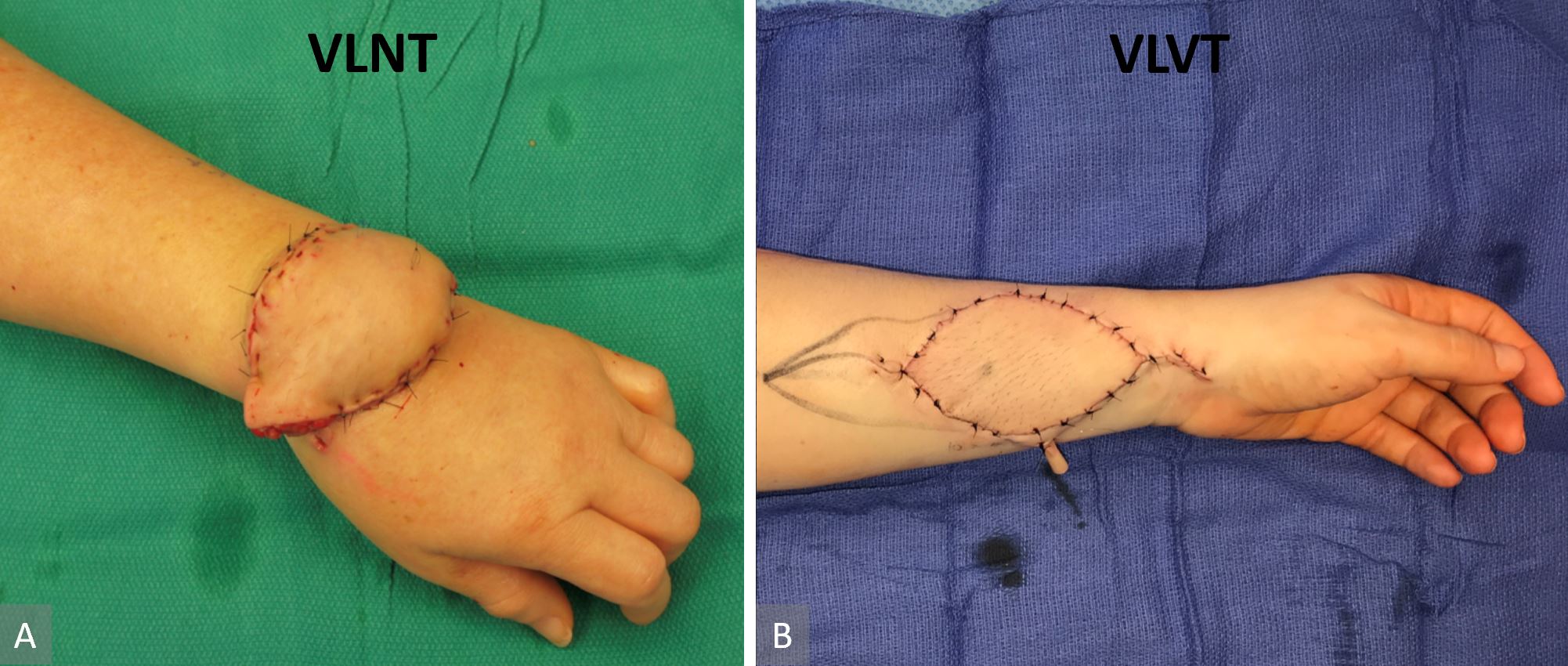
Figure 5. Comparison of lymph node flap inset and lymph vessel flap inset. Contour deformity was not avoidable due to the associated tissue bulk in a lymph node flap. In this case, we could not complete the flap inset due to venous congestion from pedicle compression with complete flap inset (panel A). The thin lymph vessel flap in combination with recipient site skin excision facilitated inset that did not disrupt anatomic contour (panel B).
Postoperative Care
The recipient limb was elevated for a week. Bandage compression of the recipient limb and ambulation for patients with lower extremity disease began on the second postoperative week. On the fourth week, all patients resumed CDT and began wearing newly fitted 30-40 mmHg compression garments for sixteen hours a day. Weaning of compression garment began after six months under the supervision of the patients’ therapists.
Outcome Evaluation
All patients were evaluated at preoperatively, and at 3-, 6-, and 12-month postoperatively with patient report, examination by the surgeon, circumference measurements, and indocyanine green lymphography. Circumference measurements were performed at elbow crease, and two levels above and below at 5 cm interval for arms, and at popliteal crease and two levels above and below at 10 cm interval for legs. Indocyanine green lymphography was performed with 0.25% indocyanine green with our standardized technique previously described [28]. Data were analyzed using Students T tests. Statistical analysis was performed with Stata 15.1 (StataCorp, College Station, Texas, USA). Unless specified, all statistical testing assumes paired two tail t-test with P < 0.05 considered as statistically significant.
Six patients with fluid-predominant limb lymphedema underwent SCIP-based VLVT (Table 1). Five had Campisi stage III and 1 had Campisi stage II disease. All were deemed inappropriate candidates for LVA due to absence of “linear” or presence of “diffuse” pattern on ICG lymphography. All were female aged 41-69, all failed CDT, and all refused vascularized lymph node transfer due to risk of donor site lymphedema. Four had upper and 2 had lower extremity disease. Duration of disease at the time of surgery ranged 2-27 years. For upper extremity disease, the side with more robust SCIA vascular anatomy seen on computed tomography angiography was selected as donor site. For lower extremity disease, the contralateral groin was used.
All flap survived, and 1 patient had (< 5%) partial flap loss which was successfully managed with local wound care. Postoperative follow-up ranged 13-27 months. All began to experience notable edema reduction and relief of symptoms (including pain, paresthesia, rigidity, and decrease range of motion) early in the postoperative course at 1-3 weeks postoperatively. Additional, progressive improvements were seen in all patients over time. At 6 months following surgery, two patients were able to greatly reduce compression garment use to only wearing the garment when heavy exertion was expected. At 1 year out, another patient (total of 3 of 6 patients) achieved the state of minimal use of compression garment of 1-2 days per week. None was able to completely stop using compression. All demonstrated stable improvements at 1 year out based on ICG lymphography and statistically significant reduction in circumference measurements (Table 1). None developed donor site lymphedema, both clinically and when screened with ICG lymphography postoperatively (Figure 6). All patients were satisfied with the surgical outcomes.
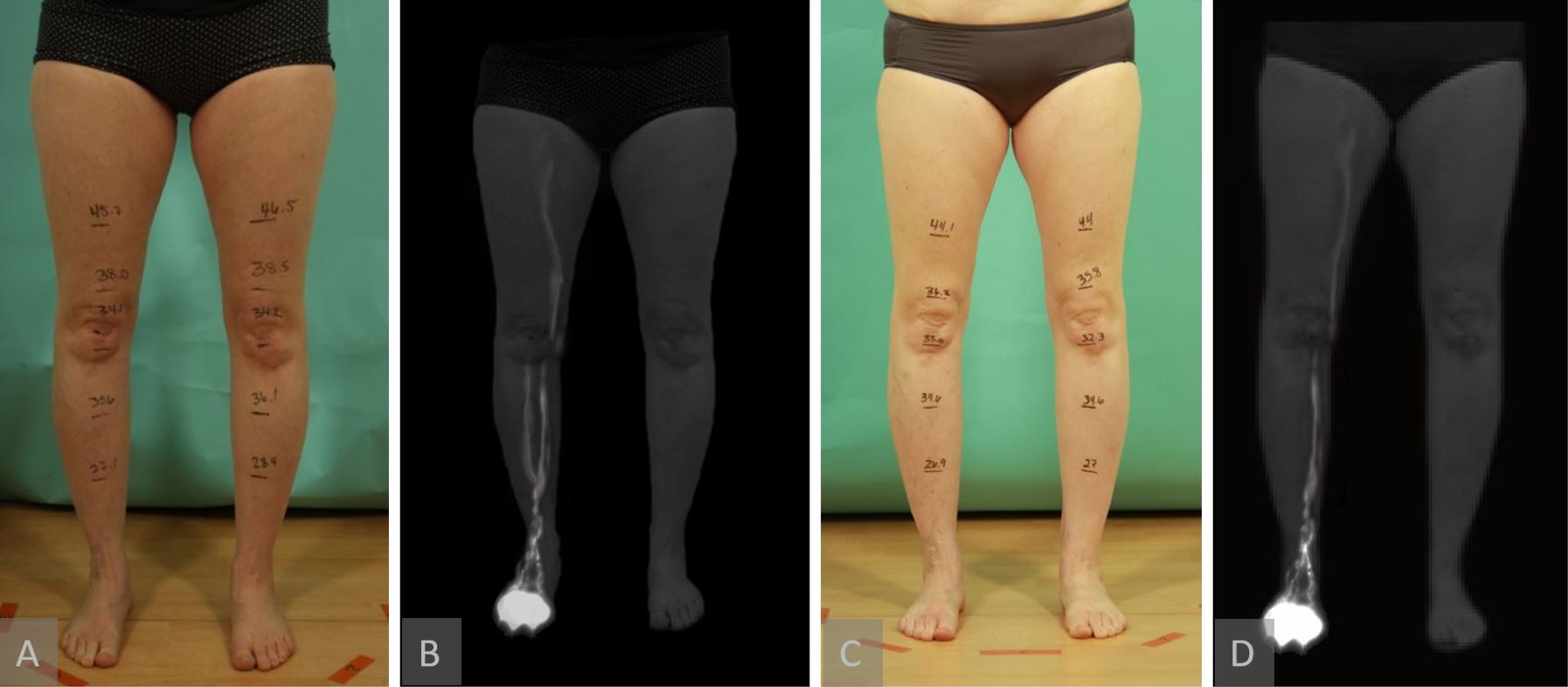
Figure 6. A representative patient showing no lymphographic injury patterns seen on postoperative ICG lymphography. This patient underwent right groin SCIP-based VLVT transferred to her arm. Clinical and lymphographic pictures, respectively (panel A and B). Postoperative pictures showing no swelling in the donor right leg and no lymphographic injury seen (panel C and D). ICG, indocyanine green; SCIP, superficial iliac artery perforator; VLVT, vascularized lymph vessel transfer.
Case 1
A 41-year-old female presented with post-mastectomy left arm lymphedema which started immediately following bilateral mastectomies for left breast carcinoma, left axillary lymph node dissection, chemotherapy, and radiation therapy. Her symptoms included diffuse hand/arm edema, pain, and paresthesia. She underwent delayed bilateral breast reconstruction with implant for her right breast and implant/latissimus dorsi flap for her left breast. This patient was a therapist and had been performing CDT herself. Upon presentation to our center, she reported a 5-year history of progressive worsening of her arm edema and wrist/forearm pain, despite rigorous CDT. Her diagnostic workup confirmed Campisi stage II, fluid-predominant disease with ICG lymphography showing “stardust” and “diffuse” pathologic patterns extending from her hand to shoulder (Figure 7A). SCIP-based VLVT was performed with a 0.4 mm flap arterial pedicle anastomosed end-to-side to a 1.3 mm radial artery, and a 2.9 mm flap venous pedicle anastomosed end-to-end to a 0.9 mm radial vena comitante (Figure 8). The patient was discharged home on postoperative day 5. The patient experienced prompt relief of her lymphedema symptoms immediately following the surgery. During the 6 months following the surgery, she noted progressive improvement in arm edema, pain, and paresthesia. She also experienced increased exercise tolerance of her left arm. She began to swim regularly, which she was unable to do before surgery. At her one-year follow-up visit, the patient reported further functional improvements. She now wore the compression garment selectively only on warm days or when expecting heavy exertion. Her reports of improvement correlated well with her significant reductions in circumference measurement (Figure 9) and the favorable changes seen on her postoperative ICG lymphography (Figure 7B).
Case 2
A 54-year-old female presented with acquired left leg lymphedema following hysterectomy, pelvic lymph node dissection, and chemotherapy for uterine carcinoma. Her symptoms included lower abdominal, left hip, and leg edema, generalized discomfort in the affected areas, and having an average of 2 cellulitis episodes a year. Although the patient had a moderately favorable response to CDT, she desired surgical intervention because she considered the requirement of lifelong compression garment use unacceptable. Her workup confirmed Campisi stage II, fluid-predominant disease with ICG lymphography showing “linear” pattern only in her foot and “diffuse” pattern in her thigh (Figure 10A). SCIP-based VLVT was performed with a 0.7 mm flap arterial pedicle anastomosed end-to-side to a 1.8 mm posterior tibial artery, and a 0.8 mm flap venous pedicle anastomosed end-to-end to a 2.0 mm posterior tibial vena comitante. The patient experienced immediate postoperative relief of her lymphedema symptoms. She was discharged on postoperative day 5. The patient experienced progressive improvements and relief of all symptoms during the first 6 postoperative months and was able to significantly wean the compression garment use during the second 6 postoperative months. At her one-year follow-up, the patient reported no abdominal/hip/limb edema or discomfort and no cellulitis. She also demonstrated correlating favorable limb circumference reduction (Figure 11) and ICG lymphographic changes (Figure 10B). She was only wearing her compression garment selectively.
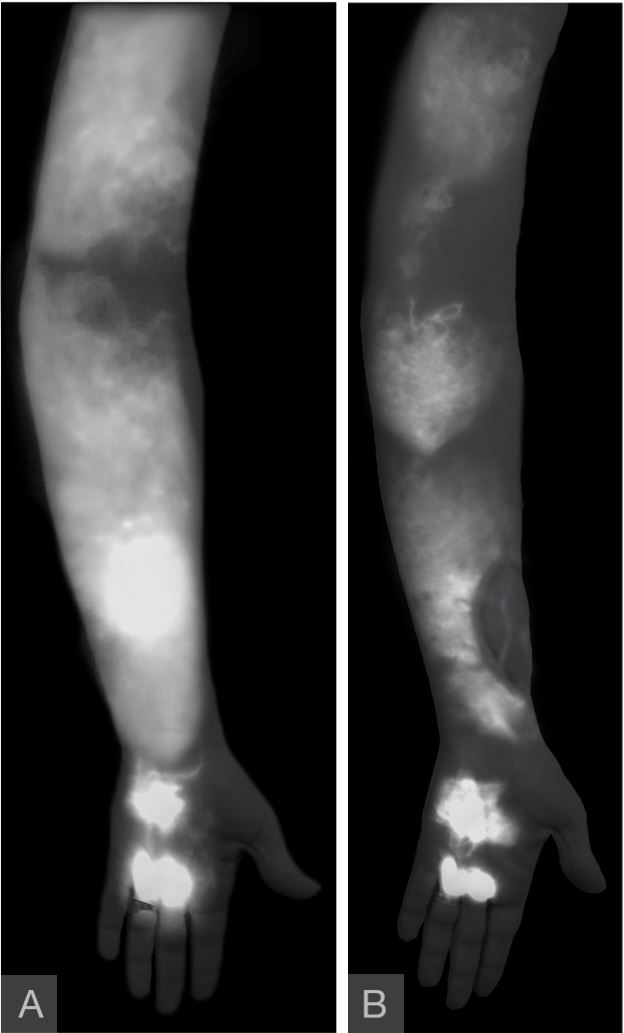
Figure 7. Severe dermal backflow patterns were seen in hand, forearm, and upper arm (panel A). The absence of “linear” pattern contraindicated LVA procedure. A year following surgery, improvements in the pathologic patterns were seen throughout the arm ( panel B). The disease patterns were notably less dense, suggestive of decreased extravasation of the contrast. “Linear” patterns were seen in the VLVT flap, indicating postoperative formation of spontaneous lymphatico-lymphatic anastomosis. LVA, lymphaticovenular anastomosis;VLVT, vascularized lymph vessel transfer.
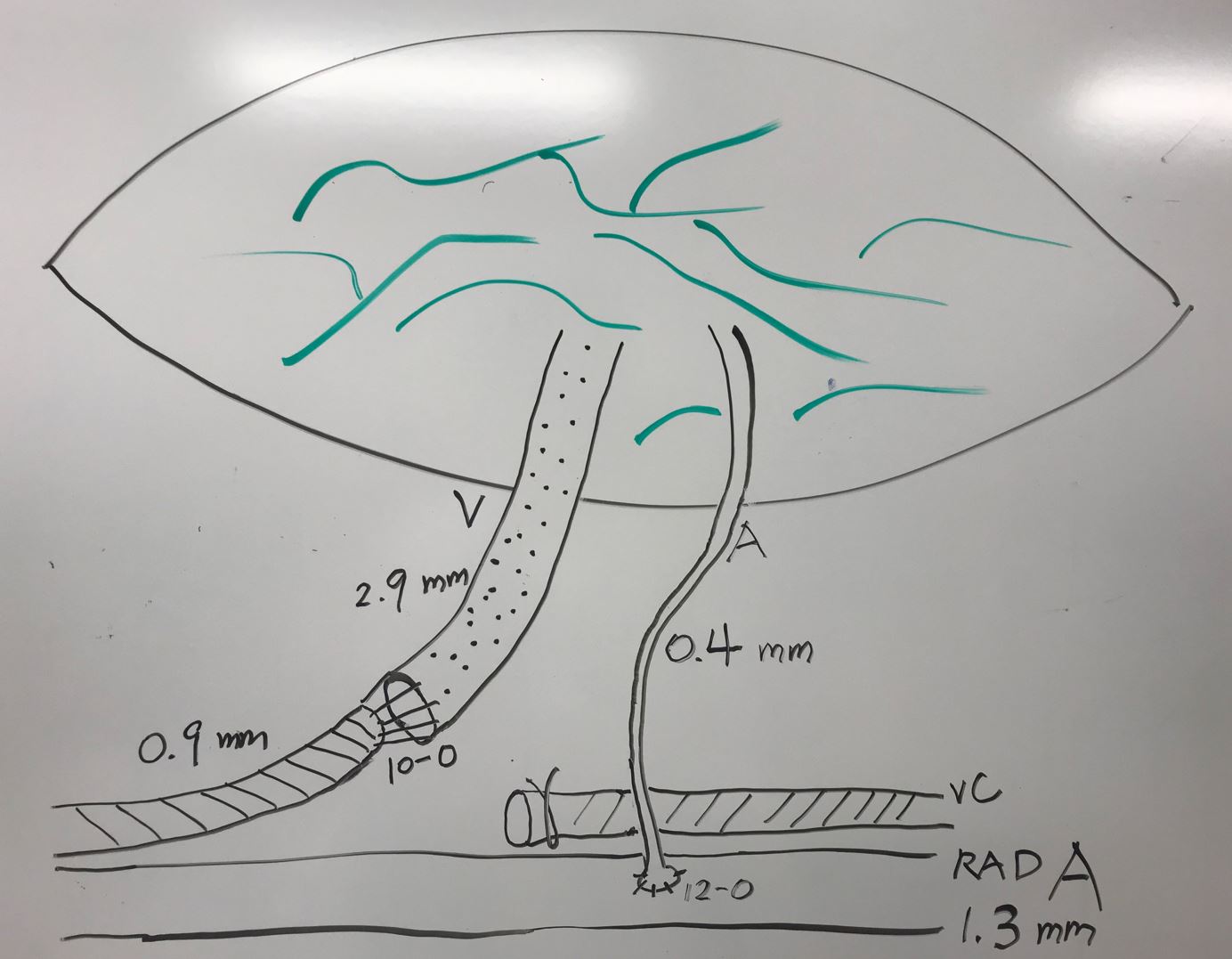
Figure 8. SCIP-based VLVT flap frequently had small arterial pedicle requiring supermicrosurgical (< 0.8 mm) anastomosis, especially if the pedicle was not dissected to its origin from the femoral artery. In our experience, a small artery (in this case 0.4 mm) was frequently sufficient to perfuse the thin VLVT flap. The flap venous pedicle was commonly sizable, except when vena comitante was used instead of the superficial circumflex iliac vein. SCIP, superficial iliac artery perforator; VLVT, vascularized lymph vessel transfer.
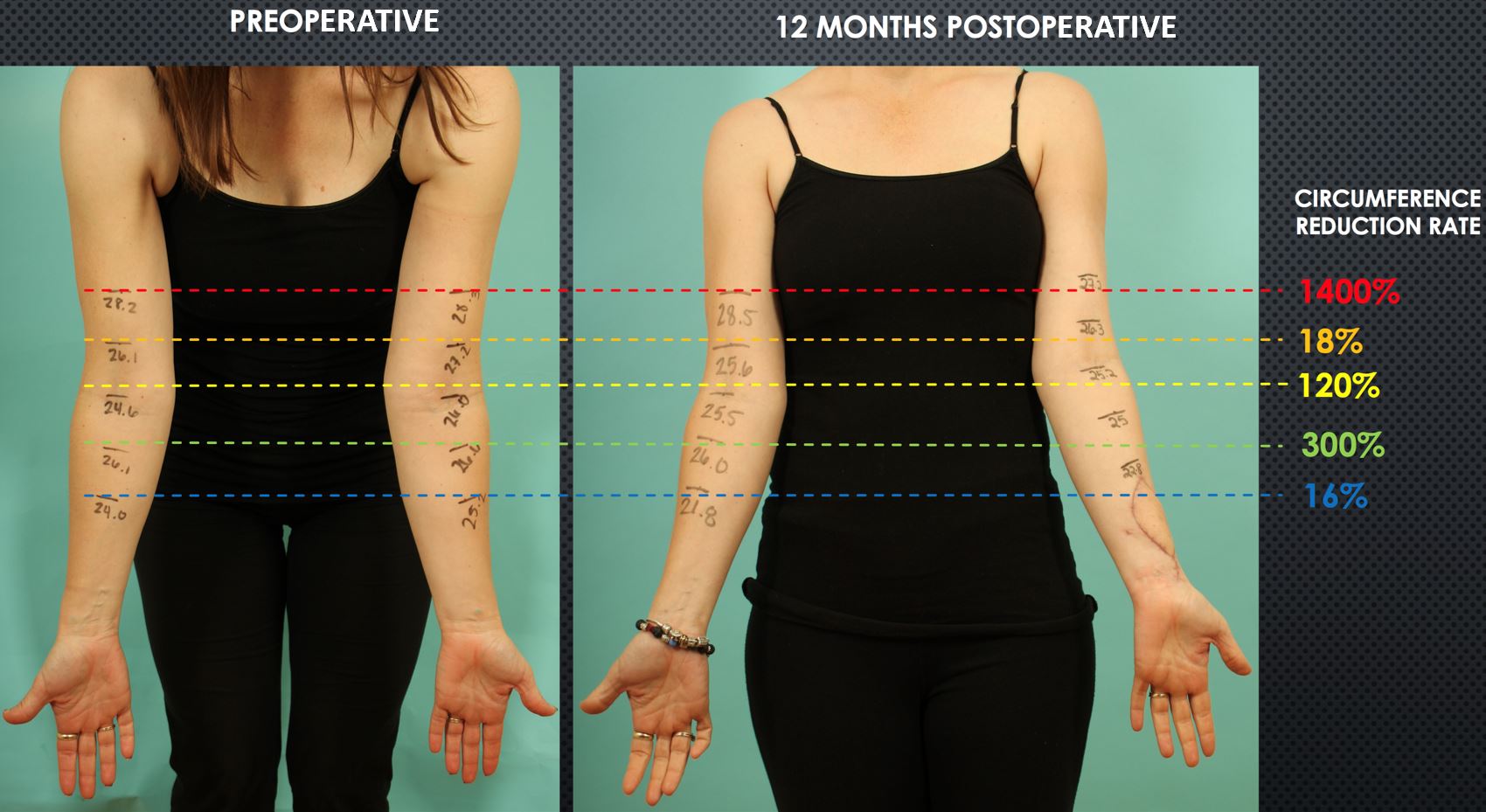
Figure 9. Postoperative reduction in arm circumferences was evident at one year from surgery when comparing preoperative (panel A) to postoperative measurements ( panel B). The reduction rates varied depending on the arm levels. Interestingly, the upper arm, which was the farthest from the VLVT flap, had the most significant circumference reduction. Circumference reduction rate=[ (a-b)-(c-d) ] / (a-b) where a= preoperative lesion arm, b=preoperative healthy arm, c= postoperative healthy arm.
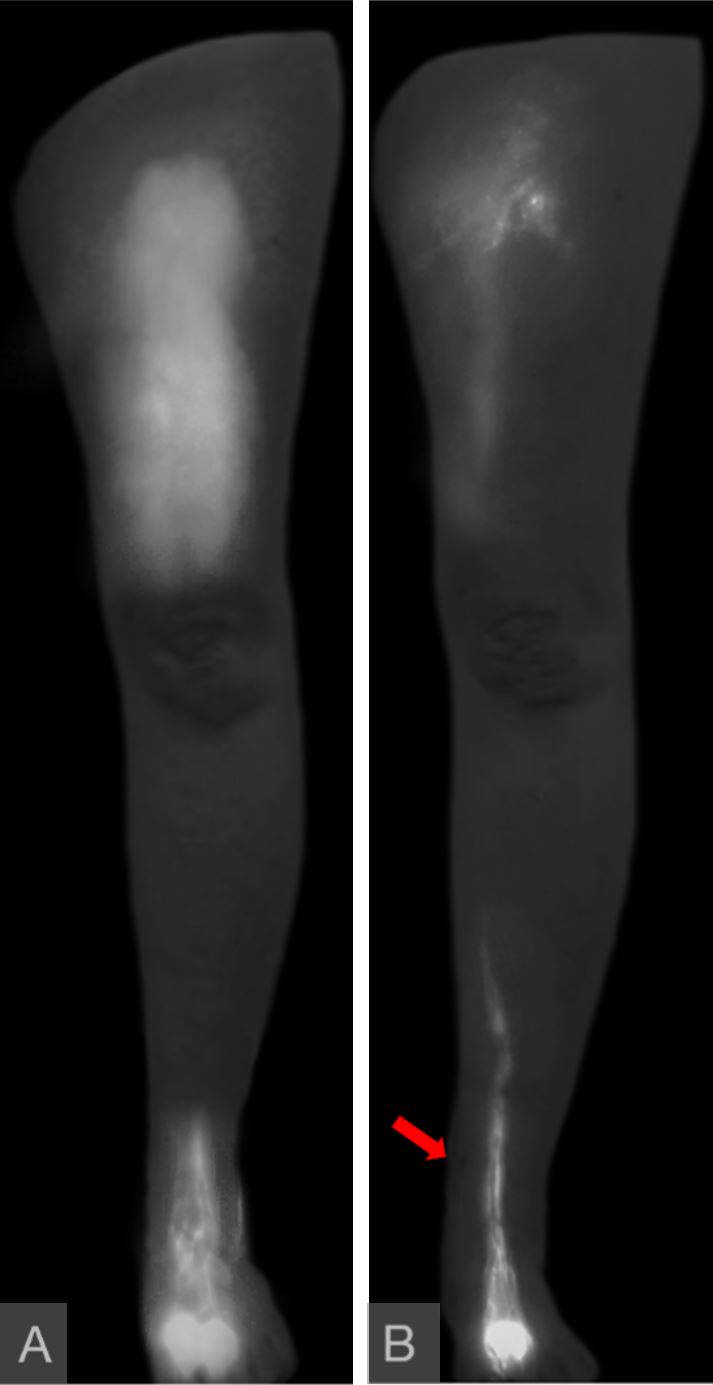
Figure 10. “Diffuse” pattern was seen in the thigh, with “linear” pattern limited to the foot (panel A). A year following surgery, the thigh “diffuse” pattern had partially resolved, and the “linear” pattern now extended further proximally in the leg (panel B). These favorable lymphographic changes correlated with patient report and circumference reduction. The flap was located immediately superior to the medial malleolus as indicated by the red arrow. The flap lymph vessels were not enhanced due to them belonging to different lymphosomes than those draining the injection sites.
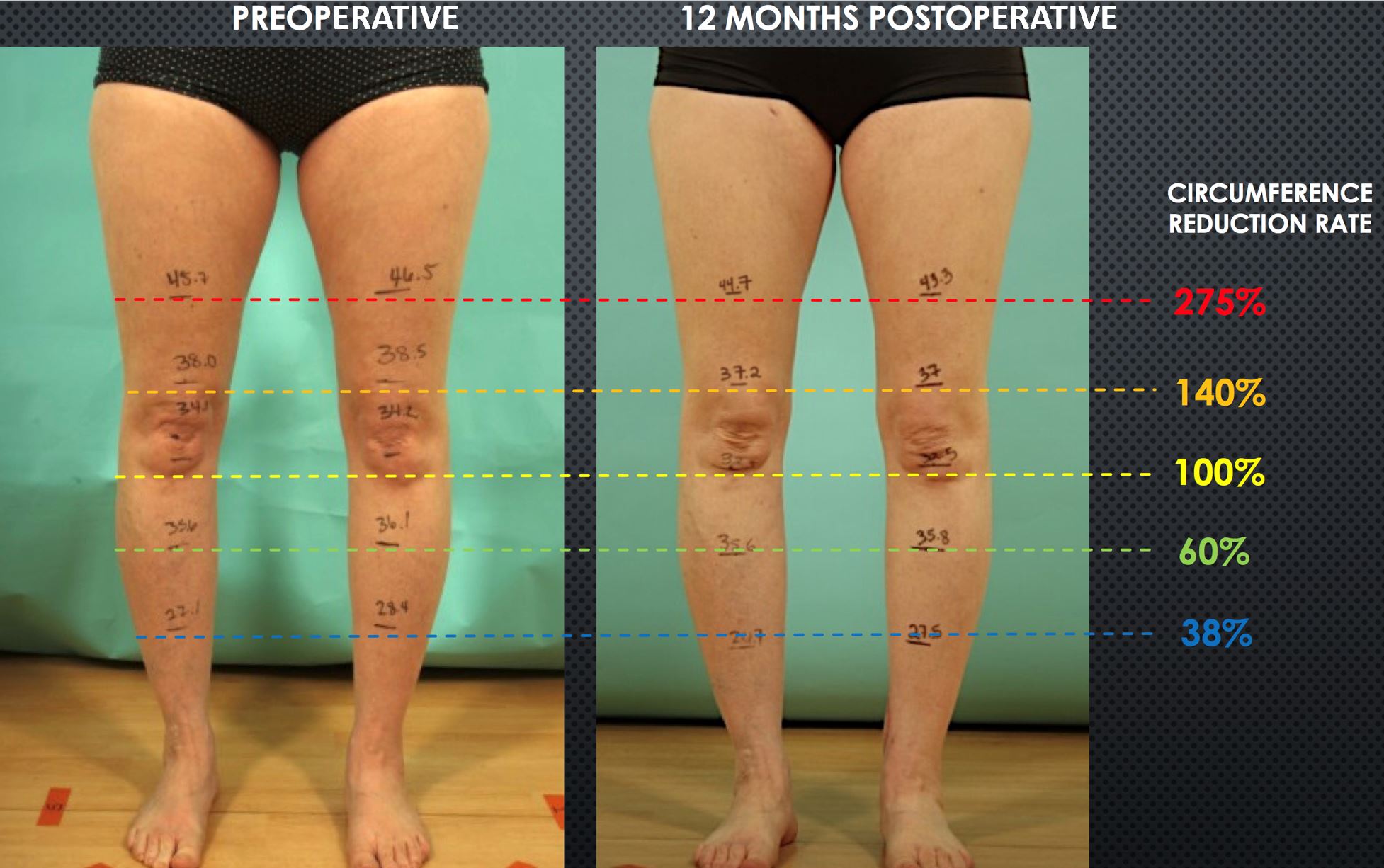
Figure 11. Postoperative reduction in leg/thigh circumferences at one year from surgery. Shown are preoperative (A) and postoperative (B) measurements. Again, the reduction rates varied depending on the levels, and the highest reduction rate was seen at the most proximal level. Circumference reduction rate=[ (a-b)-(c-d) ] / (a-b) where a= preoperative lesion leg, b=preoperative healthy leg, c= postoperative healthy leg
Vascularized lymph node transfer is considered by most to be the procedure of choice for patients with advanced, fluid predominant extremity lymphedema [29-34]. While technical refinements over the years have made VLNT a safer procedure, the risk of donor site lymphedema is only lowered and not eliminated [12,13,35-38]. Recent developments of intra-abdominal lymph node transfers promised to resolve the risk of donor site lymphedema but exposed the patients to complications associated with intraperitoneal surgery [26,38].
Previously, we offered VLNT for patients with late fluid-predominant disease who were not candidates for LVA. While we did not observe donor-site lymphedema, it remained a dreaded potential complication that caused significant anxiety in both the patients and the surgeons. Furthermore, once the excitement of initial improvement waned, many patients complained about contour deformity created by the bulky lymph node flap. Combining the above with the predicament of patients refusing VLNT after learning about the risk of donor site lymphedema, we began to seek alternative treatment.
Koshima et al. proposed the concept of lymph vessel-only vascularized transfer based on first dorsal metatarsal artery (FDMA) to treat extremity lymphedema and reported favorable outcomes [27]. The study went on to hypothesize the functioning smooth muscles lining the healthy lymphatic vessels contracting and helping to transport accumulated lymph fluid into the venous system. We had favorable experience with the FDMA-based VLVT and observed results consistent with what Koshima et al reported. However, our patients also frequently had donor wound breakdown (Figure 12). Thin SCIP-based VLVT is theoretically a superior source of vascularized lymph vessels and donor site because 1) its anatomy is familiar to plastic surgeons, 2) it contains higher lymph vessel density than the FDMA donor site, and 3) it has a track record of safety as demonstrated by Hong et al. and others [37,39-44]. Since groin lymph nodes are anatomically situated deep to the superficial fascia, flap harvest superficial to the superficial fascia ensures preservation of the lymph nodes. Another advantage of the SCIP-based VLVT is superior cosmesis. By excising a size-matched area of recipient site skin prior to insetting the thin VLVT flap, the contour deformity classically associated with VLNT and the need for secondary debulking [45] are prevented. For the above-mentioned reasons, we preferred the SCIP-based VLVT over the FDMA-based VLVT.
During dissection of the SCIP-based VLVT, groin superficial fascia can be identified as a thin, fibrous plane between the superficial and deep fat layers. The plane is particularly distinct in the lateral aspect adjacent to the anterior superior iliac spine. The two fat layers can be distinguished by observing the relative fat lobule sizes with the superficial fat lobules being smaller and more densely packed. The dissection can be performed under ICG lymphographic guidance to ensure nodal preservation and recruitment of only lymph vessels [46]. Under ICG lymphography, numerous lymph vessels can be seen coursing in the superficial fat. This compares favorably to the FDMA-based VLVT which contains 3-4 lymph vessels [27]. It was not uncommon to find the superficial circumflex iliac artery substantially smaller than 0.8 mm, especially if the arterial pedicle dissection was not extended to its femoral artery takeoff. This required supermicrosurgical anastomosis. Size mismatch was also frequently encountered in both arterial and venous anastomoses, resulting in the need for perforator-to-perforator or end-to-side anastomosis, and the inability to use a venous coupler. Proper supermicrosurgical instrument and surgeon preparation are recommended.
Like VLNT, the therapeutic mechanism of VLVT is uncertain. In VLNT, preliminary evidence suggested pump mechanism and lymphangiogenesis mediating the observed clinical improvements [4,6,7]. Theoretically, since VLVT transfers healthy, contractile lymph vessels lined with functioning smooth muscles [27], the pump mechanism of VLNT is also likely to be present in the VLVT. Spontaneous formation of lymphatico-lymphatic anastomosis (LLA) had previously been reported following standard free tissue transfer[47]. Indeed, despite not surgically performing LLA during procedures such as finger replant and deep inferior epigastric perforator for breast reconstruction, lymphedema has not been reported following these cases. It is logical to assume that spontaneous LLA also takes place following VLVT. Koshima et al. proposed VLVT mediating its effect by draining excess lymph in the affected limb into the venous system via the healthy vascularized lymph vessels [27]. In their experience, the FDMA-based VLVT was efficacious in 80% of the patients who had previously failed LVA [27]. In theory, SCIP-based VLVT, with its significantly higher lymphoid density than the FDMA-based VLVT, may provide higher therapeutic efficacy.
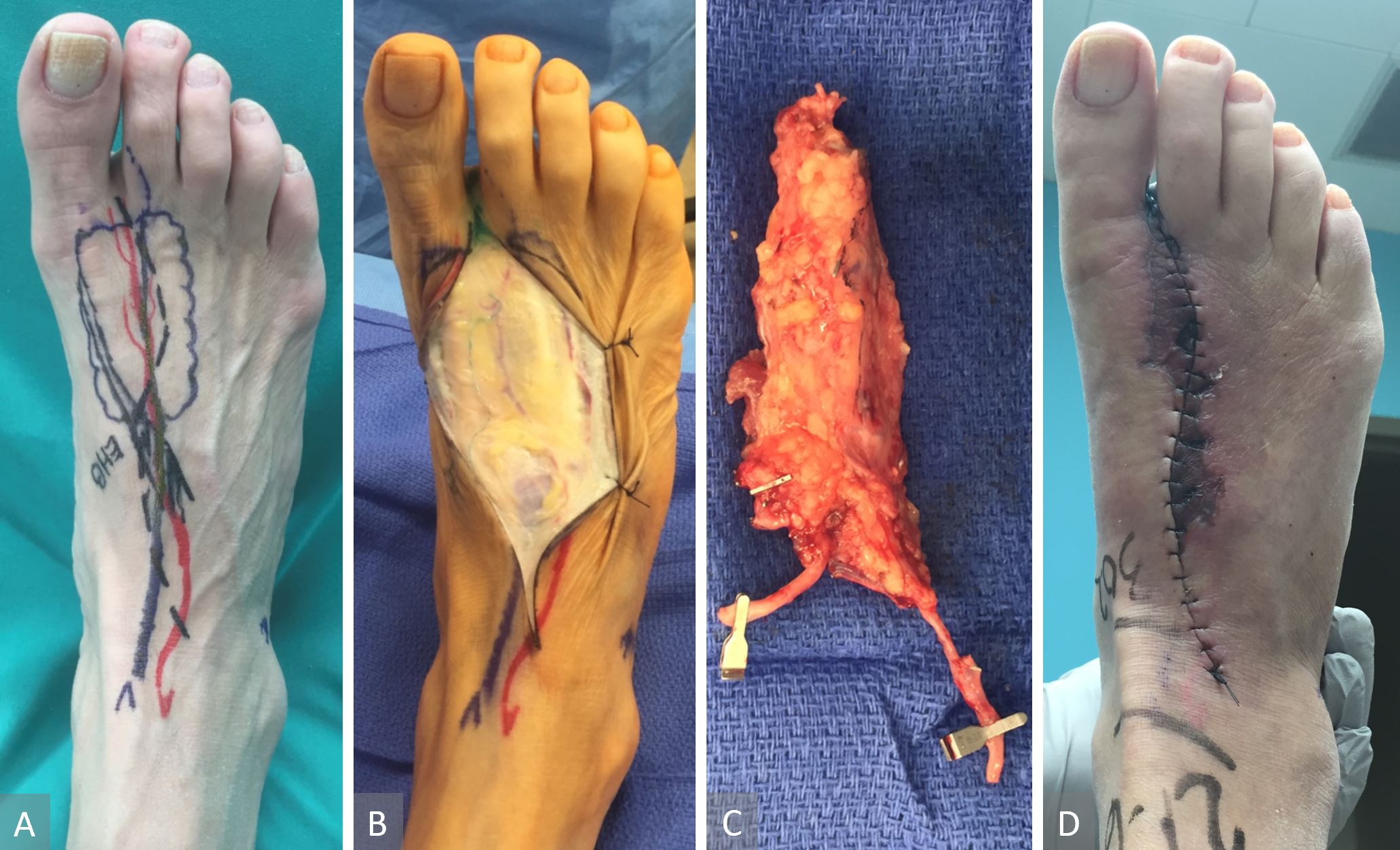
Figure 12. FDMA-based VLVT flap. Flap marking ( panel A). This flap included 3-4 perivascular lymph vessels around the FDMA. One of the perivascular lymph vessels can be seen here ( panel B). Complete harvest of the FDMA-based VLVT flap. Note the vein shown was a superficial vein that did not drain the flap. Instead, the flap was drained by FDMA’s accompanying veins ( panel C). Donor wound breakdown frequently occurred due to the skin over the first intermetatarsal space being devascularized from the flap harvest ( panel D). FDMA, first dorsal metatarsal artery; VLVT, vascularized lymph vessel transfer.
Vascularized lymph vessel transfer demonstrated promising results in treating extremity lymphedema without the inclusion of nodal tissue. SCIP-based VLVT using the thin SCIP flap harvest technique may potentially offer higher therapeutic efficacy than the FDMA-based VLVT. Our experience suggested the feasibility of the inclusion of SCIP-based VLVT in the reconstructive microsurgeon’s armamentarium for treating extremity lymphedema.
Received date: July 13, 2019
Accepted date: September 20, 2019
Published date: October 31, 2019
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2019 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Video Abstract
Authors report a case of lower extremity lymphedema treated by LVA that preoperatively mapped not only lymphatic vessels by PDE, but also veins and venules using Veinsite™ .
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
ICG lymphography is an invaluable tool in lymphedema management. Both immediate and delayed scans are needed when performing the study. The delayed scan needs to be performed at the time of the lymphographic plateau to appreciate the full extent of the pathology. Using a recumbent cross trainer, the lymphographic plateau can be achieved in 15 minutes following ICG injection. We have found this exercise enhanced ICG lymphography protocol worthwhile of adoption by high volume lymphedema centers to raise diagnostic accuracy and efficiency.
This article holds critical relevance for healthcare professionals, particularly in the fields of microsurgery, oncology, and vascular medicine. It thoroughly examines the diagnostic challenges faced in distinguishing between recurrent lymphedema and deep vein thrombosis in elderly cancer patients following lymphovenous anastomosis surgery. It highlights the significant risk of misdiagnosing deep vein thrombosis as lymphedema, a mistake that can delay critical treatment due to their clinical similarities. The case study of a 79-year-old patient emphasizes the importance of a comprehensive reassessment, considering the patient's entire medical history, including the effects of cancer treatments like immunotherapy. The article stresses the need for a holistic approach to patient management and the utilization of advanced diagnostic tools for accurate diagnosis and treatment. It is essential reading for its insights into the complex dynamics of postoperative care and the critical importance of accurate diagnosis in treating elderly cancer patients effectively.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
Osteoarthritic finger joints are often repaired with joint implants, arthrodesis, or a vascularized interphalangeal joint graft. However, grafts can damage the donor toe. Based on the results of this study, the authors suggest that vascularized distal interphalangeal joint transfers from the second toe may be suitable for reconstructing these defects through microsurgery.
The authors reviewed the MDCT images to show the number of lymph nodes superior to the saphenofemoral junction. In this study, on average, 3.67 nodes existed. However, there were 4 percent of cases with no countable nodes. This result indicates that appropriate preoperative screening is needed for this procedure.
In the manuscript entitled ”Vascularized Lymph Vessel Transfer For Extremity Lymphedema – Is Transfer Of Lymph Node Still Necessary?” Wei F. Chen and coauthors reported their early results of transferring vascularized lymphatic tissue to treat advanced lymphedema. The paper is well written regarding their surgical techniques. Their results revealed significant improvement with resumed linear pattern of lymphatic regeneration after surgery, in particular the presence of lymphatic vessel inside the flap. A photo showing the donor site and confirmed no lymphedema in the donor site will make the paper even more informative.
ResponseWe have followed the request to include a picture showing the condition of the donor site.
This is an informative study and provide a easy alternative possible method to treat the Lymphedema. The manuscript is well written.
Chen WF, McNurlen M, Ding J, Bowen M. Vascularized lymph vessel transfer for extremity lymphedema - Is transfer of lymph node still necessary? Int Microsurg J 2019;3(3):1. https://doi.org/10.24983/scitemed.imj.2019.00119