Vertical sleeve gastrectomy (VSG) is a type of bariatric surgery that is accepted as the simplest but most efficient surgical modality to treat morbid obesity. A mouse model of VSG has been widely utilized to investigate the underlying mechanisms of VSG. There are numerous microsurgical techniques for VSG in mice, and we report herein a modified technique with 2 disposable micro-clamps to simplify the surgery. The key aspects of our modification are to use the micro-clamps as non-crushing tissue clamps for anastomosis as well as vascular clamps for hemostasis. Mean body weight at postoperative week 1 was 24.1 grams, which is equivalent to a 16.1% loss of original body weight, and there was no mortality after antrum preservation VSG. This simplified surgical technique will best serve as a tool to investigate either the underlying mechanisms of VSG or the intrinsic change to the stomach after VSG.
Video. This technique is a modified version of the mouse vertical sleeve gastrectomy using two disposable microclamps.
Vertical sleeve gastrectomy (VSG) is one of the most common forms of bariatric surgery, and its beneficial effects on morbid obesity and accompanying comorbidities have widely been accepted [1]. Its efficacy and safety have widely been proven, but its underlying mechanisms that lead to weight loss and metabolic improvements are still controversial [2]. Most researchers studying such mechanisms use a mouse model of VSG, and there have been various surgical techniques to perform VSG in mice [3]. However, some techniques required tedious, time-consuminng continuous sutures along the gastric wall before gastrectomy, and some techniques used metal clips which were left permanently in the abdomen [4,5]. To overcome such demerits and to simplify the existing techniques, we describe herein a modified technique of mouse VSG using 2 disposable micro-clamps.
All experimental protocols have been approved by the University of Nevada, Reno Institutional Animal Care and Committee (protocol #00727).
Preoperative Preparation
All laboratory mice were purchased from Jackson Laboratories (Bar Harbor, ME, United States). Male C57BL mice, aged 8 weeks, and with a body weight 28 to 30 grams were used for VSG. Mice were fed ad libitum and were placed on a liquid-only diet (chocolate-flavor Boost®, Nestle, Vevey, Switzerland) 3 days before surgery. During the liquid diet period, cages were embedded with paper cellulose particles (Alpha dri, Shepherd Specialty Papers, Milford, NJ, United States) to minimize pica.
VSG in Mice
Prior to surgery, the mouse was weighed to obtain a baseline weight measurement. The surgical room was equipped with a state-of-the-art anesthesia system, heated water bath, and heating pad set to 37°C, to keep the animal warm during surgery and recovery after anesthesia. Warm saline solution (0.9%) was used for irrigation. Prior to any surgical procedures, the surgical field was cleaned with 70% ethanol, and proper surgical techniques were used to open the autoclaved surgery pack and dissecting equipment. The mouse was anesthetized using an induction chamber with 5% isoflurane and an oxygen (O2) flow rate of 1 L/min. The absence of a hind-limb pinch-withdrawal reflex was verified to ensure the proper degree of anesthesia. Following this, the mouse was maintained with 1% to 3% isoflurane and an O2 flow rate of 0.6 L/min. Eye ointment was placed on the animal’s eyes to keep them moist during surgery. An analgesic (buprenorphine 1 mg/kg) was also administered subcutaneously. Hair from the umbilicus to the xyphoid process was shaved. Following shaving, the abdominal skin was prepared with povidone-iodine and 70% alcohol. The surgical field was prepared by placing sterile surgical drapes on either side of the mouse, and autoclaved tin foil was used to create a surgical drape for the mouse abdomen. A small hole was cut in the tin foil to allow access to the abdomen area. An incision in the skin from the mid-abdomen (umbilicus) to the level of the xiphoid cartilage was made using an iris scissors, the linea alba was identified, and a cut was made through the body wall along the linea alba. Cotton tip applicators (CTAs) were used to gently elevate the stomach out of the abdomen and the greater omentum was removed from the greater curvature by blunt dissection. Following this, the short gastric artery that runs between the fundus of the stomach and the spleen was divided with electrocautery. The stomach was then exteriorized from the abdominal cavity, and a gauze pad was placed under the stomach and wet with saline (Figure 1A). A small gastrotomy incision was made along the greater curvature of the corpus, and the remaining gastric contents were removed by gentle squeezing with the CTAs. For VSG with antrum preservation, the line of transection started from the angle of His and ended at the margin of the pancreas gastric lobe so that at least 80% of the gastric volume could be removed after surgery. Two micro-clamps (TKMV-1, Baxter, IL, United States; one from the angle of His and one from the pancreas margin) were applied along this line, and spring scissors were used to cut the gastric sleeve (Figure 1B and 1C). For VSG without antrum preservation, the pancreas gastric lobe was resected from the stomach to expose the greater curvature of antrum, and the micro-clamp was applied just beneath the pylorus to remove most of the antrum. The cut edge was closed with 7-0 monofilament nonabsorbable suture with a tapered needle by removing the clamp backward at intervals of 1 mm and then placing simple, interrupted stitches to close the exposed edge (see attached https://doi.org/10.24983/scitemed.imj.2020.00137 for details of the procedure). An average of 20 knots was used to securely close the stomach, and any gastric remnants were flushed with saline throughout the procedure in order to keep the tissue moist and clean. After an additional lavage, the stomach was placed back into the abdominal cavity under the liver using a CTA. The abdominal muscle layer and skin layers were closed using 6-0 monofilament absorbable suture with a tapered needle in a simple, discontinuous pattern. The mouse was then allowed to recover on a heating pad for 10 to 15 minutes before returning it to its home cage. The animal was continuously attended until it has regained consciousness and was able to move freely.
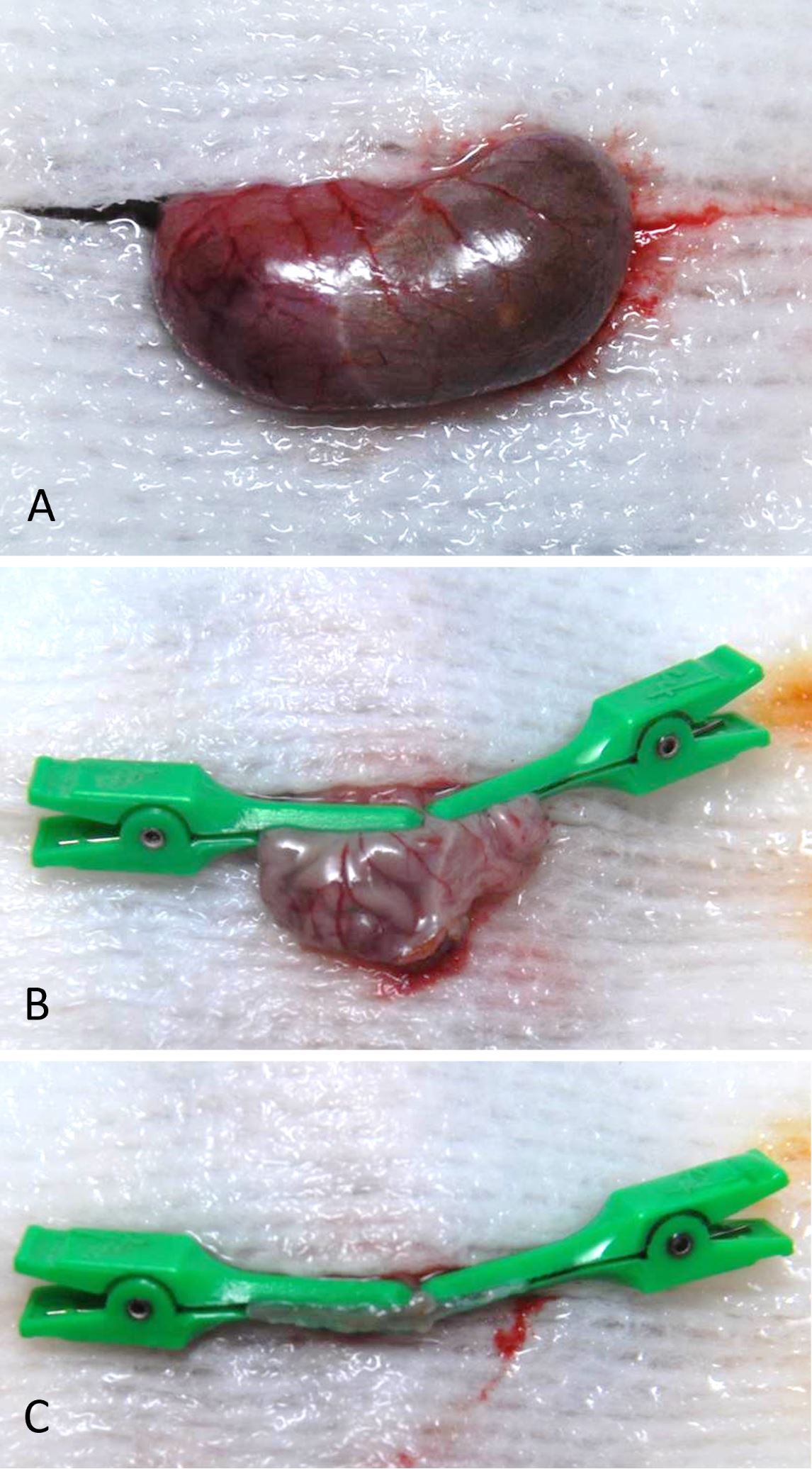
Figure 1. (A) Stomach was exteriorized after omentum clearance. Note the distended stomach after 3 days of liquid diet. (B) Two micro-clamps were applied in both directions to delineate the line of transection, and (C) the greater curvature side was removed. Note the gastrotomy for stomach evacuation.
Postoperative Mouse Care and Measurement
Following the return of the mice to their home cages, the cages were placed on a heating pad. Mice were maintained on a liquid diet for 5 days following surgery. An analgesic buprenorphine (1 mg/kg subcutaneously) was administered for 2 days after surgery. During the postoperative period, the body weight of animals was accessed on a daily basis until postoperative 7 days, and then at 2 weeks and 4 weeks thereafter. Food intake, defecation, activity level, and disposition were also accessed daily to ensure proper healing and recovery for 7 days post-surgery. In mortality cases, autopsy was performed to reveal the cause of death.
Overall, 42 mice underwent VSG (24 with antrum preservation, 14 without antrum preservation) for this study. Mice were euthanized for experiments at various times after surgery, and the stomach was harvested (Figure 2). Because of the high mortality rate and low efficiency, VSG without antrum preservation was abandoned after 14 cases, and only the 24 cases of VSG with antrum preservation were included in the final analysis.
Body Weight Changes
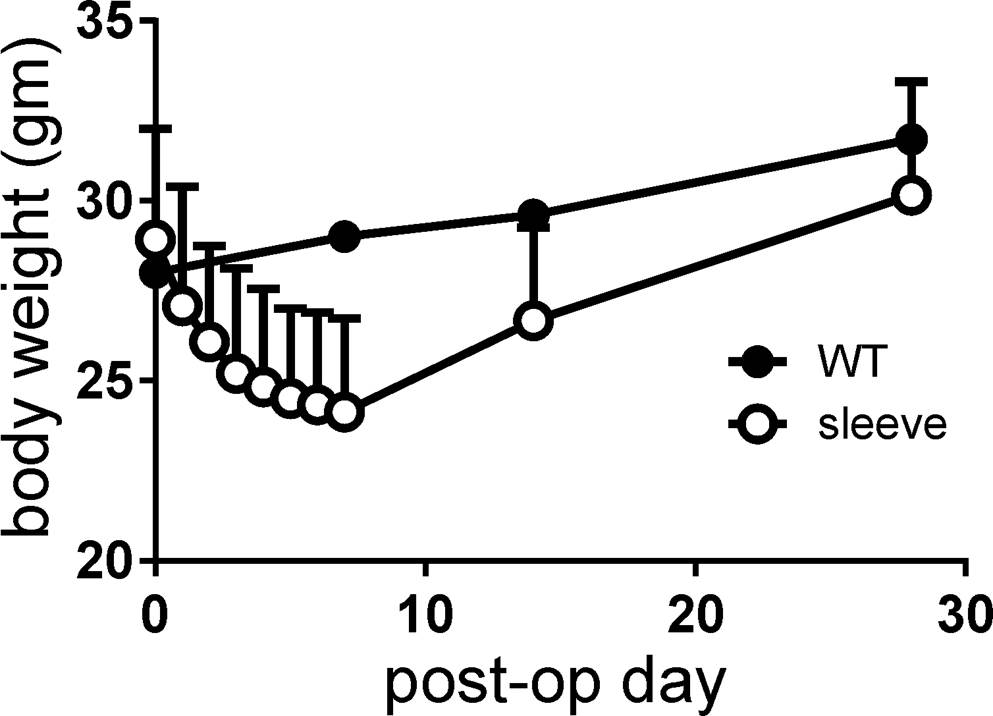
Mean body weight at the time of operation was 28.9 grams. Postoperative body weight was lowest at postoperative week 1 (mean 24.1 grams; range 20-30 grams) and recovered after that, returning to its original weight and surpassing it thereafter. Although statistically not significant, body weights of VSG mice were always lower than non-operated control mice (Figure 3).
Mortality and Complications
There were no mortalities after VSG with antrum preservation, but 9 mice (64.3%) died during the early postoperative period after VSG without antrum preservation. The cause of death was anastomosis disruption secondary to gastric outlet obstruction in 8 cases (Figure 4A) and pancreatic juice leakage in 1 case (Figure 4B).
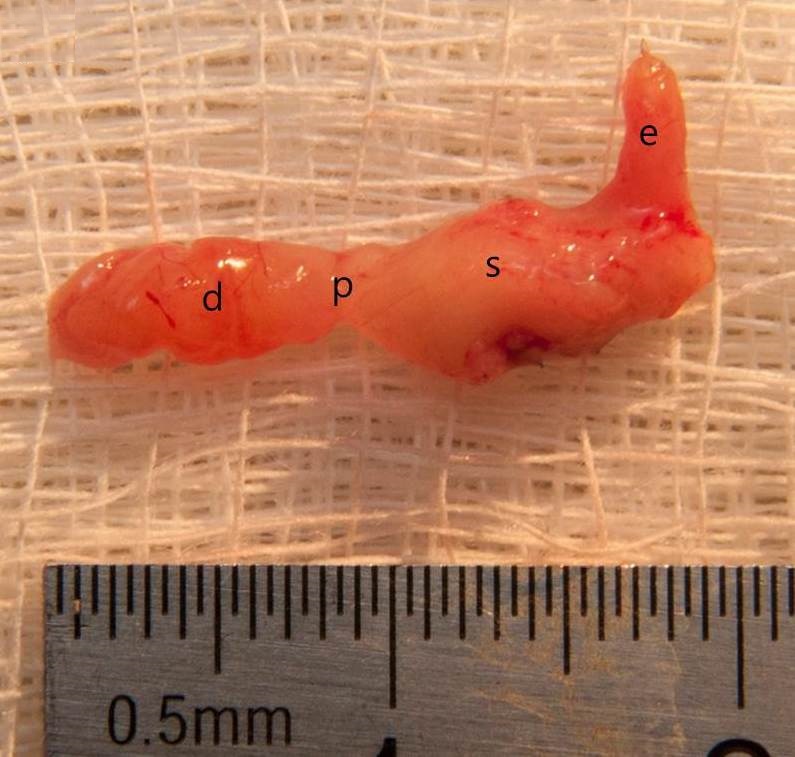
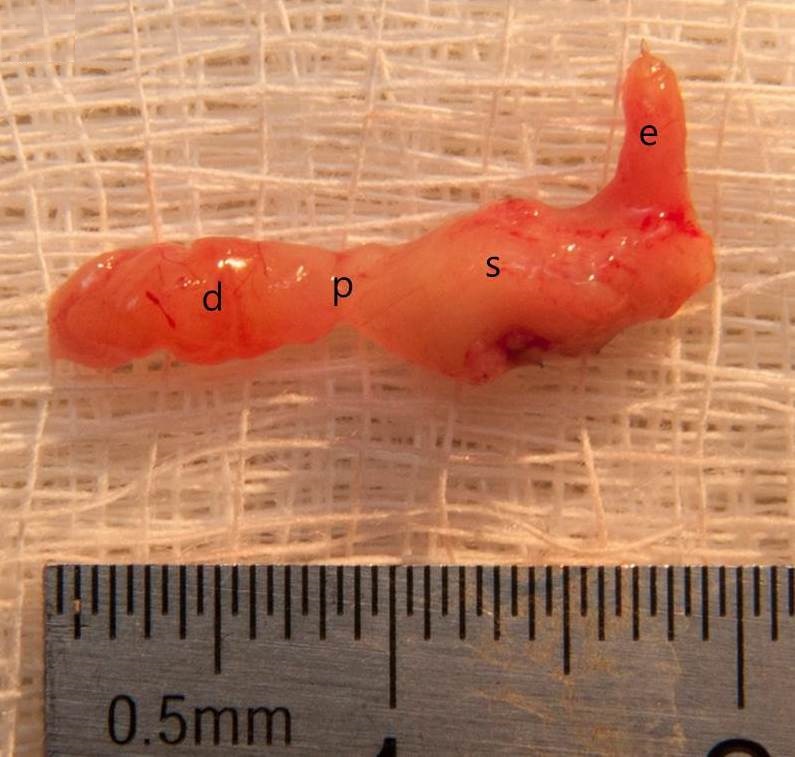
Figure 2. The vertical sleeve gastrectomy with antrum preservation specimen after 4 months of surgery (d, duodenum; p, pylorus; s, stomach; e, esophagus).
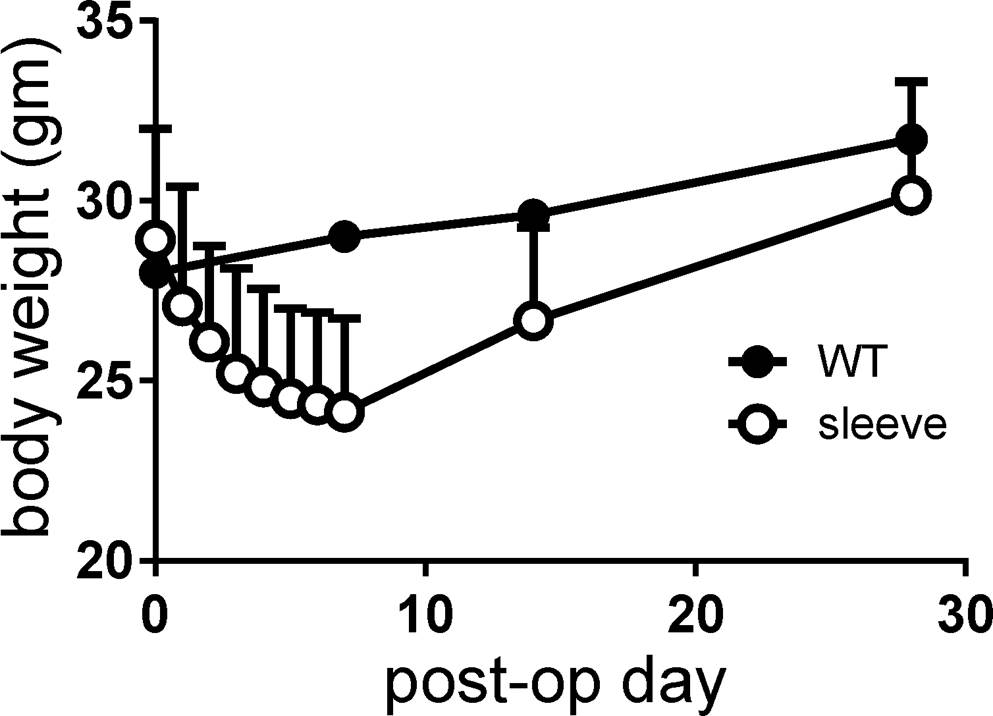
Figure 3. Body weight changes after surgery, measured on a daily basis for 1 week and then at 2 and 4 weeks. Note that the body weight of mice with vertical sleeve gastrectomy was always lower than that of control mice (wild-type, WT).
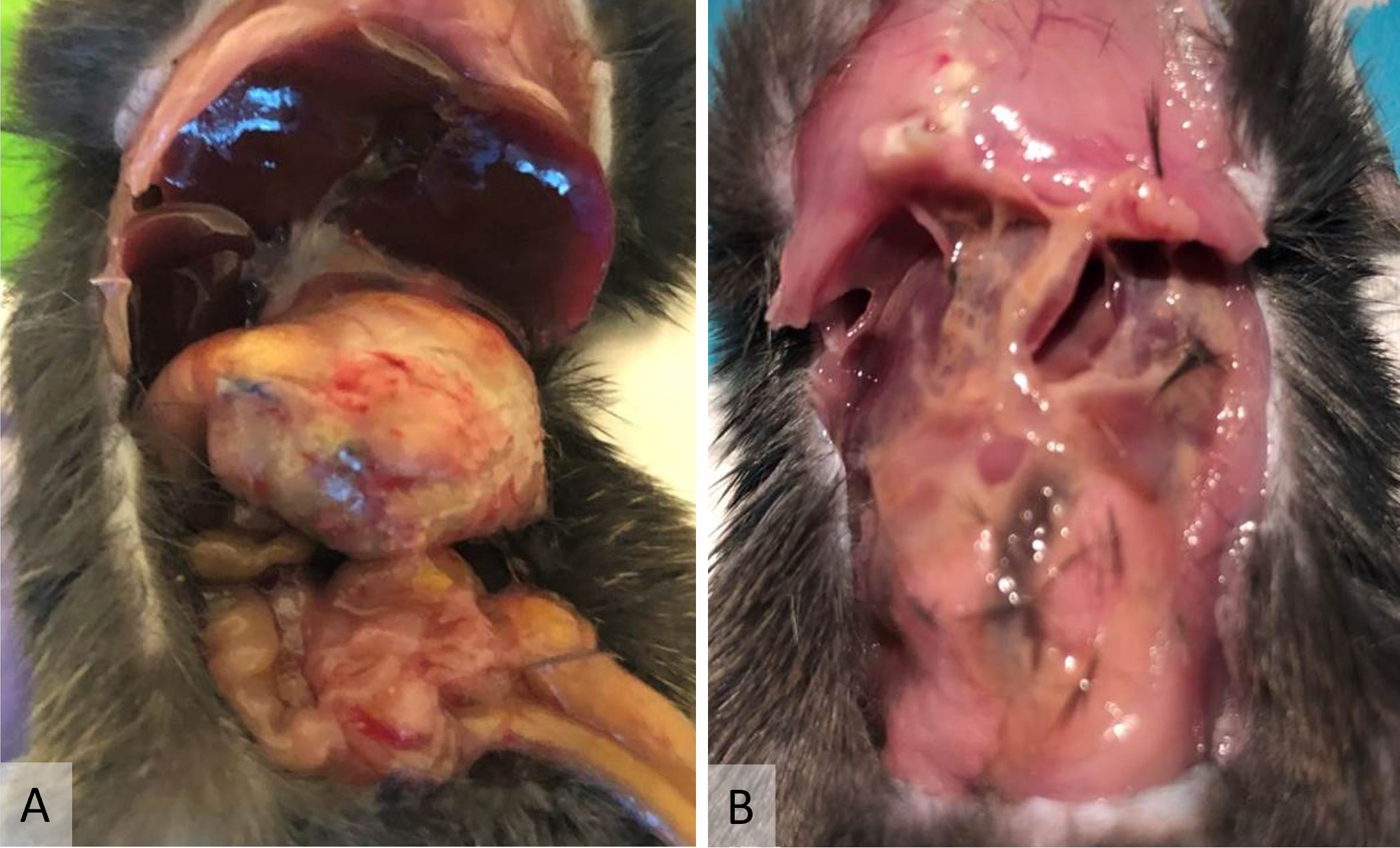
Figure 4. (A) Gastric outlet obstruction was a typical complication after vertical sleeve gastrectomy without antrum preservation. Note the severely distended stomach. This complication was occasionally complicated by anastomosis perforation. (B) This mouse died of pancreatic juice leakage after vertical sleeve gastrectomy without antrum preservation. Note the self-digestion by pancreatic juice.
The mouse VSG technique described in this paper was developed to simplify and ease existing procedures without increasing postoperative mortality or morbidity. Two micro-clamps played a key role in the surgical technique of our VSG model. Originally, the clamp used in our model was designed for clamping small veins in human vascular surgery (Figure 5). However, we found that this clamp was also useful for experimental mouse surgery. The clamp conveniently served as both a non-crushing tissue clamp and a vascular clamp for anastomosis and hemostasis in mouse VSG.
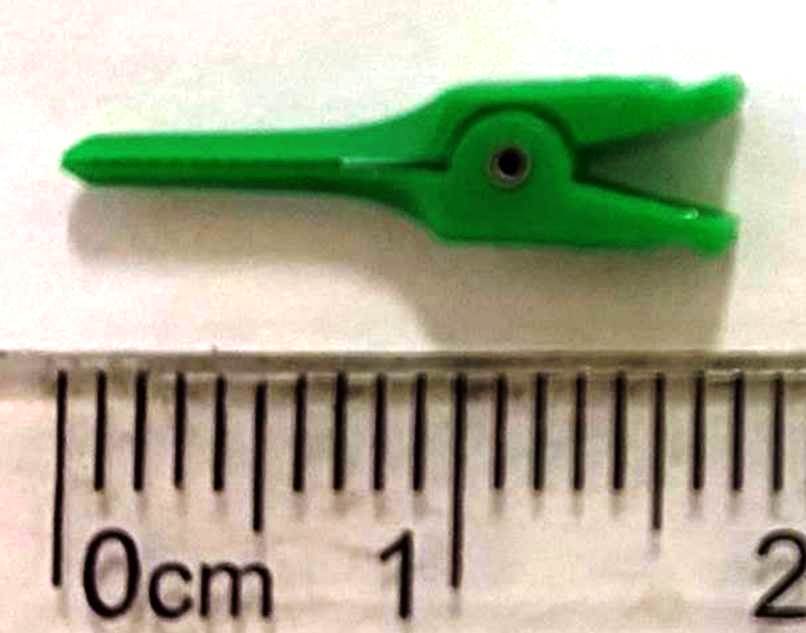
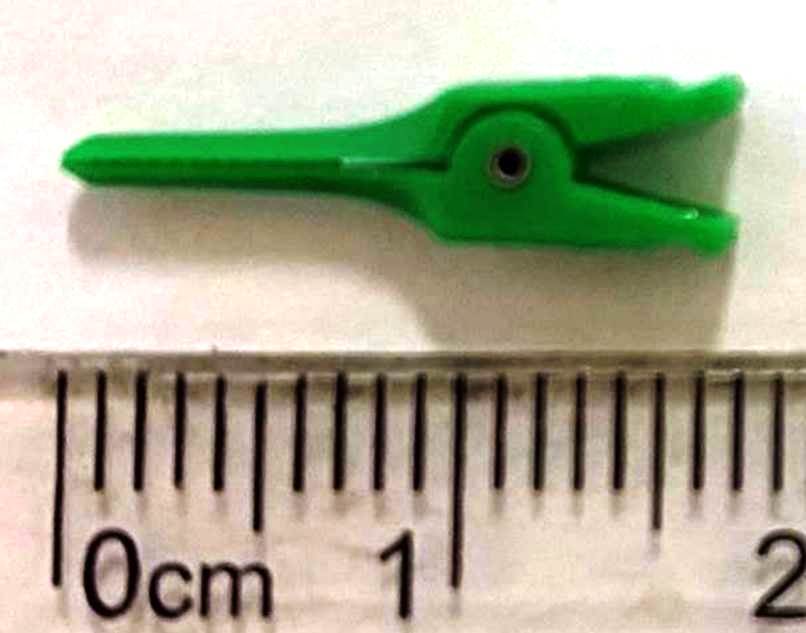
Figure 5. The plastic, disposable micro-clamp was designed for vascular surgery in humans.
Garibay et al. described a technique of placing a continuous suture along the intended line of transection to prevent spillage of gastric contents before transection [4]. Also, they had ligated prominent branches of the gastric artery and vein before transection. However, placing such sutures is a time-consuming procedure in itself, and proceeding with surgical manipulation without completely clearing the gastric contents may cause potentially fatal abdominal sepsis after surgery. In our procedure, we could prevent intraoperative spillage and bleeding by maintaining clamps on the cut edge of the stomach throughout the course of the surgery. Schlager et al. showed a rather simple technique by applying 2 permanent titanium clips, instead of suturing, to close the cut edge of the stomach [5]. This technique requires only a few sutures to close the gap between the 2 clips, and the operation time would be shorter than ours. However, permanently placing clips on the stomach may restrict the natural recovery of gastric motility after VSG. Altered gastric motility after VSG has been known to be one of the key mechanisms of VSG, and many researchers focus on gastric motility changes after VSG in mice [6]. Therefore, the surgical model using permanent clipping does not appear to be suitable for the purpose of this study. Our model leaves behind no foreign material that may hinder the recovery or changes in gastric motility after surgery. Similar to our techniques, Hao et al. used a pair of custom-made stainless steel clamps for VSG [7]. However, there is no description on the clamp cost or how they are manufactured; therefore, this technique may not be available for most researchers. The clamps used in this study are commercially readily available, cost-effective, and provide constant quality performance.
During the early phase of surgery, we tried both VSG with antrum preservation and without preservation. However, in cases of VSG without antrum preservation, there was such a high rate of complications that we abandoned the procedure after 14 cases; therefore, our result analysis only includes the antrum preservation cases. Most of the complications were related to gastric outlet obstruction, which was probably due to the transection and suturing being in close proximity to the pylorus. The mouse stomach is so small that it would be very difficult to obtain a true safety margin from the pylorus while achieving enough antrum resection. In humans, some surgeons prefer to remove the majority of the antrum for better outcomes, and they usually start the resection 2 to 3 cm from the pylorus [8]. However, in mouse VSG for study purposes, we recommend starting the resection right next to the pancreas gastric lobe so that the pylorus and pancreas remain intact after surgery.
Mouse VSG using two micro-clamps simplified existing VSG procedures by achieving field sterility and hemostasis with a single clamping.
Received date: May 10, 2020
Accepted date: September 07, 2020
Published date: September 23, 2020
This study was supported by a 2017 research grant from Kangwon National University (grant no. 520170434).
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2020 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Video This technique is a modified version of the mouse vertical sleeve gastrectomy using two disposable microclamps.
A novel technique of sequential ETS micro-venous anastomoses using three vessel loops for IJV occlusion and a single vascular clamp to retract and hold the anastomoses sites in position.
The authors describe various patient and breast-related factors that influence surgical outcomes while also addressing some techniques and principles for aesthetic microsurgical reconstruction.
The incidence of high-grade sinonasal adenocarcinomas of non-intestinal origin is extremely rare. In this case report, the authors present a very rare case of high-grade non-intestinal sinonasal adenocarcinoma presenting with a challenging diagnosis. Because of its significantly different prognosis, this study provides a detailed explanation of how the authors differentiate it from other sinonasal tumors. In addition, they describe how a radical endoscopic resection was applied in order to achieve a total excision.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
The PLOSEA technique detailed in this study addresses the significant challenge of managing large vessel size discrepancies in microvascular surgery with an innovative and accessible method. By partially obliterating the larger vessel lumen before anastomosis, the technique reduces risks of thrombosis and misalignment, simplifying the procedure without sacrificing effectiveness. This advancement is particularly valuable as it allows surgeons with varying levels of experience to perform complex reconstructions with greater confidence and improved patient outcomes. A key feature is the inclusion of a detailed video demonstration, providing a dynamic and comprehensive visual guide that surpasses traditional static images. This video meticulously elucidates each procedural step, enhancing understanding and facilitating the practical application of the technique. Emphasizing technical precision, patient safety, and surgical efficiency, this study offers a compelling narrative for medical professionals. The transformative impact of the PLOSEA technique on surgical practice underscores its importance, presenting a novel approach that can enhance the quality of care and expand the capabilities of microsurgeons worldwide.
This systematic review and meta-analysis provide a pragmatic evaluation of drain-free versus drain-based DIEP flap techniques for breast reconstruction, challenging the traditional reliance on drainage. By analyzing postoperative outcomes, the study highlights the potential for refining surgical strategies to enhance patient comfort and recovery without compromising safety. The findings offer a neutral perspective, suggesting that clinical practice may not necessarily depend on the use of drains. This revelation prompts medical professionals to reassess existing surgical approaches and may catalyze a paradigm shift in postoperative care. Presented with clear narrative and rigorous data analysis, the article encourages readers to consider the broader implications of surgical innovations on patient care protocols.
This report presents the first documented case of parotid NUT carcinoma with an NSD3::NUTM1 fusion, characterized by rapid metastasis and patient death five weeks post-surgery. This outcome challenges prior reports suggesting favorable survival for non-thoracic NSD3::NUTM1 tumors, indicating potential parotid-specific aggression. By integrating 13 previously published cases with the current case, the article provides a comprehensive clinicopathological reference for this rare malignancy. While further validation is required, the findings advocate for targeted NUT immunohistochemistry and molecular profiling in undifferentiated parotid tumors. BET inhibitors show therapeutic potential, underscoring the need for early recognition and precision-based care.
AI is rapidly evolving from a supportive tool into a core component of medical decision making and evidence synthesis, reshaping how clinicians interpret information at the point of care. Yet, while much of medical AI research emphasizes algorithmic performance and explainability, it seldom addresses the more practical question: how should physicians evaluate an AI recommendation in real-world, high-risk situations when fluent outputs can conceal critical errors. This Perspective offers a clinician-centered framework that treats AI outputs as provisional, testable hypotheses rather than definitive conclusions. By guiding users through premise verification, terminological precision, evidence appraisal, and causal analysis, it provides a structured defense against hallucinations, selective reporting, and data poisoning, using otolaryngology as a high-stakes, multimodal model. By placing clinical judgment at the center of AI use, this work shifts the field from passive automation toward safer, more accountable decision support grounded in patient safety.
Moon SB, Kim D, Koh SD. A modified technique of mouse vertical sleeve gastrectomy using two disposable micro-clamps. Int Microsurg J 2020;4(1):5. https://doi.org/10.24983/scitemed.imj.2020.00137