Background: NUT carcinoma (NC) is a rare and aggressive malignancy characterized by rearrangements of the NUTM1 gene. These rearrangements typically involve genes from the bromodomain and extraterminal protein family, such as BRD4. Primary NC of the parotid gland is exceedingly uncommon, and its molecular heterogeneity remains poorly understood. Frequently, these tumors are misdiagnosed as other poorly differentiated carcinomas. To enhance understanding of this malignancy, we performed a comprehensive literature review and present the first reported case of parotid NC harboring an NSD3::NUTM1 fusion.
Methods: A 45-year-old male presented with a rapidly enlarging parotid mass. Imaging revealed a 9.4 cm tumor with cervical lymph node involvement. He underwent total parotidectomy, radical neck dissection, and reconstruction. The tumor was analyzed by histopathology, immunohistochemistry, and targeted RNA sequencing using the Illumina TruSight Fusion Panel. A literature review of parotid NC cases was integrated with this case for comparative clinicopathological and molecular analysis.
Results: Histopathology showed a high-grade, poorly differentiated carcinoma featuring squamous differentiation, cribriform architecture, and rhabdoid morphology. Immunohistochemistry demonstrated positivity for NUT-P4, cytokeratin 7, and MOC31, with patchy cytokeratin 5/6 and P40 staining confined to squamous differentiation areas. Molecular analysis identified an in-frame NSD3::NUTM1 fusion, a novel finding in parotid NC. Despite aggressive surgical intervention, the patient developed extensive pulmonary and hepatic metastases within six days after surgery and died due to metastatic disease five weeks postoperatively. Analysis of 13 previously reported cases and the current case yielded a median patient age of 33 years, with 35.7% initially misdiagnosed as squamous cell carcinoma or undifferentiated carcinoma. Most cases exhibited squamous differentiation and demonstrated positive nuclear staining for NUT on immunohistochemistry. Metastases were frequently observed, involving lymph nodes in 64.3% of cases, bones in 42.9%, and the lungs or liver in 21.4%. The BRD4::NUTM1 fusion was predominant (50%), while the NSD3::NUTM1 fusion correlated with rapid disease progression. Overall survival durations varied significantly among cases, ranging from 1.25 to 47 months, with a median survival of approximately seven months.
Conclusions: This first reported case of parotid NUT carcinoma with an NSD3::NUTM1 fusion underscores its aggressive clinical course and diagnostic complexity. The rapid metastatic progression contrasts with earlier reports of non-thoracic NSD3::NUTM1 tumors, suggesting site-specific prognostic differences. Accurate diagnosis relies on NUT immunohistochemistry and molecular confirmation. Comprehensive genomic profiling and evaluation of targeted therapies are essential to improve outcomes in this lethal entity.
Nuclear protein in testis (NUT) carcinoma (NC) is a rare and aggressive malignancy characterized by chromosomal rearrangements involving the NUTM1 gene, typically fused to bromodomain and extraterminal (BET) family genes such as BRD4 or BRD3 [1,2]. NC predominantly arises in midline anatomical structures, including the thorax and the head and neck regions [3]. The prognosis is generally poor, with median overall survival ranging from 6.5 to 6.7 months [1,2]. Although NC primarily originates at midline sites, recent studies indicate it can also develop in non-midline locations, such as salivary glands [4,5]. Additionally, emerging research has uncovered significant molecular heterogeneity within NC, exemplified by the identification of the ZNF532::NUTM1 fusion variant, thereby broadening our understanding of its pathogenesis [4]. These developments highlight the need for further investigation into the clinical and molecular characteristics of rare NC subtypes, particularly those affecting the salivary glands.
Knowledge Gaps in Parotid NC Research
Despite advancements in NC research, our understanding of salivary gland NC, particularly in the parotid gland, remains significantly limited. Existing literature has predominantly focused on the BRD4::NUTM1 fusion, with limited exploration of other molecular variants such as NSD3::NUTM1 [4,6]. Additionally, the lack of epidemiological data specific to parotid NC restricts our comprehension of its true incidence and geographical distribution, further obscuring the understanding of its rarity [5]. Misdiagnosis of parotid NC as poorly differentiated carcinoma or squamous cell carcinoma is common, reflecting inadequate diagnostic criteria and awareness among clinicians [4,5]. Moreover, inconsistencies in the histopathological descriptions within the existing literature, combined with a lack of systematic morphological analyses, hinder the development of standardized diagnostic approaches [5]. Previous literature reviews on parotid NC have been limited in scope and depth, offering insufficient clinicopathological and molecular detail. Hence, there is an urgent need to integrate a broader array of case data and provide structured comparative analyses to improve the understanding of molecular diversity, diagnostic challenges, and research implications of parotid NC.
Research Objectives
To address these knowledge gaps, this report describes a patient diagnosed with primary parotid NC harboring the NSD3::NUTM1 fusion. To our knowledge, this is the first documented occurrence of this particular fusion variant in parotid NC, thereby enhancing the understanding of this underrecognized malignancy. The primary rationale for this case report is the tumor’s distinct molecular characteristics, which may improve diagnostic accuracy and clinical management. By thoroughly documenting the histopathological, immunohistochemical, and molecular findings, and conducting a detailed literature review, this study aims to elucidate diagnostic complexities and the molecular heterogeneity of parotid NC. Specifically, our objectives are to: (1) describe the clinicopathological and molecular characteristics of this novel NSD3::NUTM1 fusion case; (2) perform a comprehensive comparative analysis with previously reported parotid NC cases; and (3) provide insights that could lead to standardized diagnostic criteria, thereby advancing future research and clinical practice.
A 45-year-old male was referred to the head and neck oncology service of a tertiary medical center for evaluation of a rapidly enlarging soft tissue mass located in the unilateral postauricular region. His medical history included severe obstructive sleep apnea, chronic obstructive pulmonary disease, a prior pulmonary embolism, and a successfully treated hepatitis C infection. Histopathological analysis of the initial biopsy specimen demonstrated a poorly differentiated squamous cell carcinoma.
Preoperative Imaging Findings
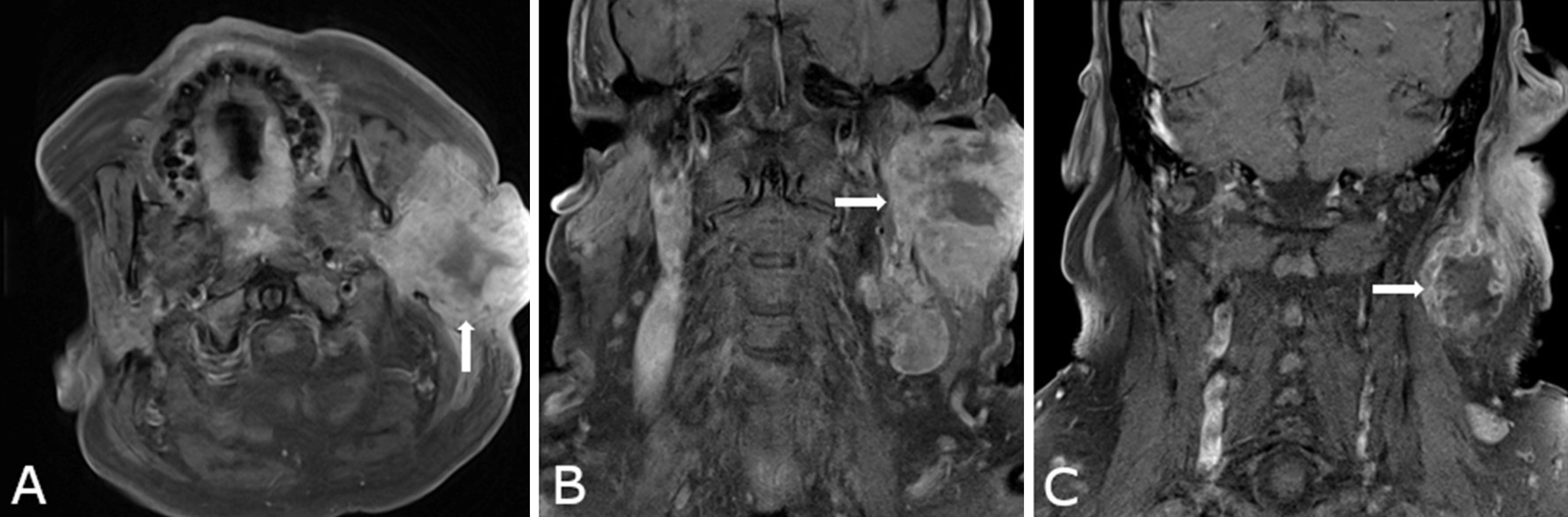
Preoperative imaging, including computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI), revealed an ill-defined mass centered in the superficial lobe of the parotid gland, with extension into the deep lobe. The lesion measured 44 × 59 × 60 mm at initial evaluation (Figures 1A–B). Suspicious cervical lymphadenopathy was identified on the ipsilateral side (Figure 1C); however, no evidence of distant metastasis was observed. Follow-up imaging performed two months later demonstrated substantial tumor enlargement. The mass had increased to 90 × 94 × 90 mm in size, representing a nearly fivefold increase in volume. Surgical resection was subsequently performed due to the rapid progression.
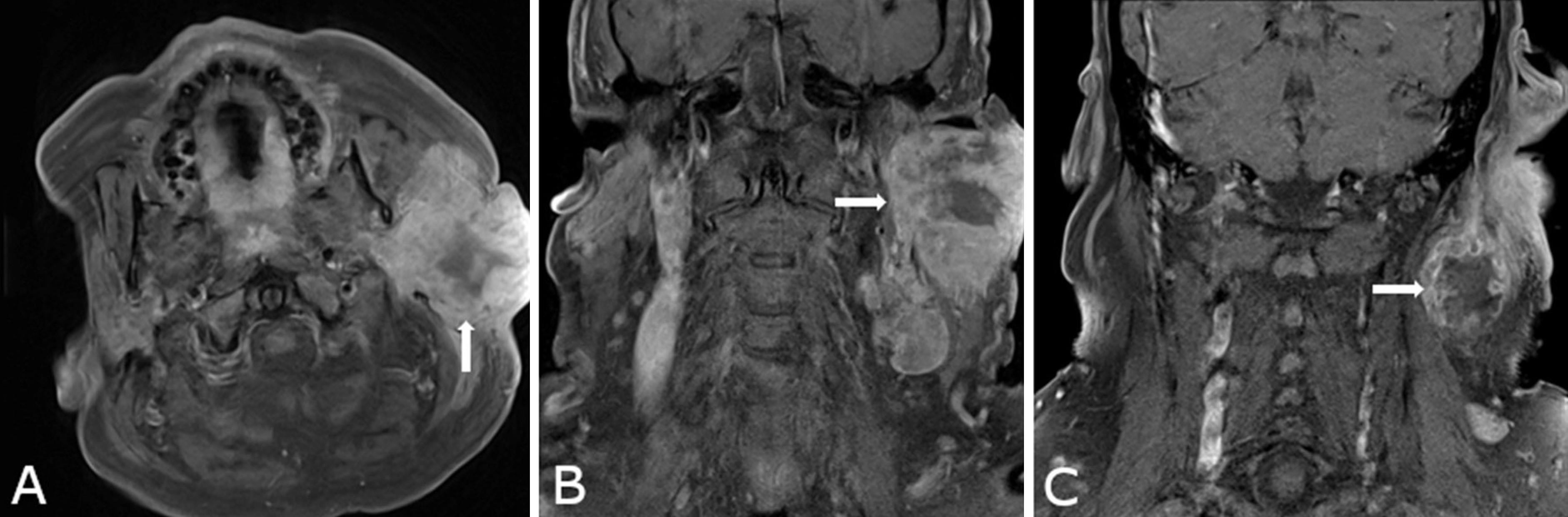
Figure 1. Preoperative contrast-enhanced magnetic resonance imaging (MRI) for anatomical delineation of the parotid gland tumor. (A) Axial and (B) coronal T1-weighted fat-saturated images demonstrate an ill-defined mass centered in the superficial lobe of the parotid gland, exhibiting medial extension into the deep lobe (arrows). The lesion shows peripheral contrast enhancement and internal T1 hypointensity, radiologically consistent with central necrosis. (C) An enlarged adjacent cervical lymph node is visualized, also demonstrating peripheral enhancement and central necrosis (arrow), suggestive of nodal metastatic involvement.
Surgical Procedure
The patient underwent a left total pinnectomy, followed by a total parotidectomy with deliberate sacrifice of the facial nerve due to tumor involvement. Additional procedures included a partial mandibulectomy with condylotomy and a radical neck dissection. Surgical reconstruction was achieved using an anterolateral thigh free flap, along with static facial reanimation utilizing fascia lata slings.
Pathology Findings
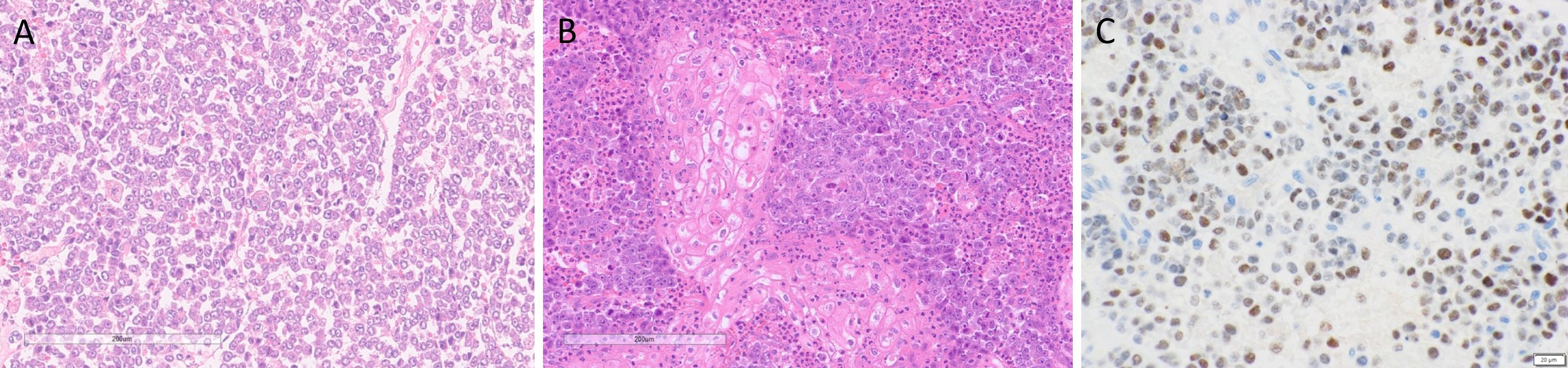
Macroscopically, the fungating tumor measured 90 × 75 × 80 mm in the superior to inferior, anterior to posterior, and superficial to deep dimensions. The cut surface was variegated, featuring firm white nodules interspersed with softer hemorrhagic regions. Histological analysis revealed an invasive carcinoma exhibiting high-grade morphological features. Tumor cells exhibited discohesion and nuclear enlargement, with an absence of overt differentiation (Figure 2A). Foci of squamous differentiation were noted, along with poorly formed glands and cribriform architectural patterns. Focal areas of keratinizing squamous differentiation were observed, accompanied by dense neutrophilic infiltration (Figure 2B). Additional findings included focal spindle cell proliferation, areas of cytoplasmic clearing, and rhabdoid morphology. Tumor cells also displayed marked nuclear pleomorphism, prominent nucleoli, and variable amounts of eosinophilic cytoplasm. Lymphovascular invasion was observed, while no perineural invasion was identified. Metastatic carcinoma was present in 42 of 47 dissected lymph nodes, with extranodal extension documented in at least four. The postoperative pathological stage was determined to be pT4aN3bM0, indicating extensive local invasion, significant regional lymph node involvement with extranodal extension, and no evidence of distant metastasis at the time of surgery.
Immunohistochemistry Results
Immunohistochemical analysis demonstrated strong cytoplasmic positivity for cytokeratin 7 and patchy expression of cytokeratin 5/6, confined to areas of squamous differentiation. P40 showed sparse nuclear staining in regions with squamous features. Extensive nuclear positivity for NUT-P4 was observed, demonstrating diffuse nuclear reactivity within tumor cells, confirming the diagnosis of NC (Figure 2C). Membranous staining for MOC31 was also noted, including within squamoid areas. P16 exhibited a patchy mosaic pattern. Expression of BRG1 and INI1 was retained, while CD56 showed rare focal positivity. Immunostains for androgen receptor protein, myogenin, synaptophysin, chromogranin, INSM1, DOG1, SOX10, smooth muscle actin, and caldesmon were negative.
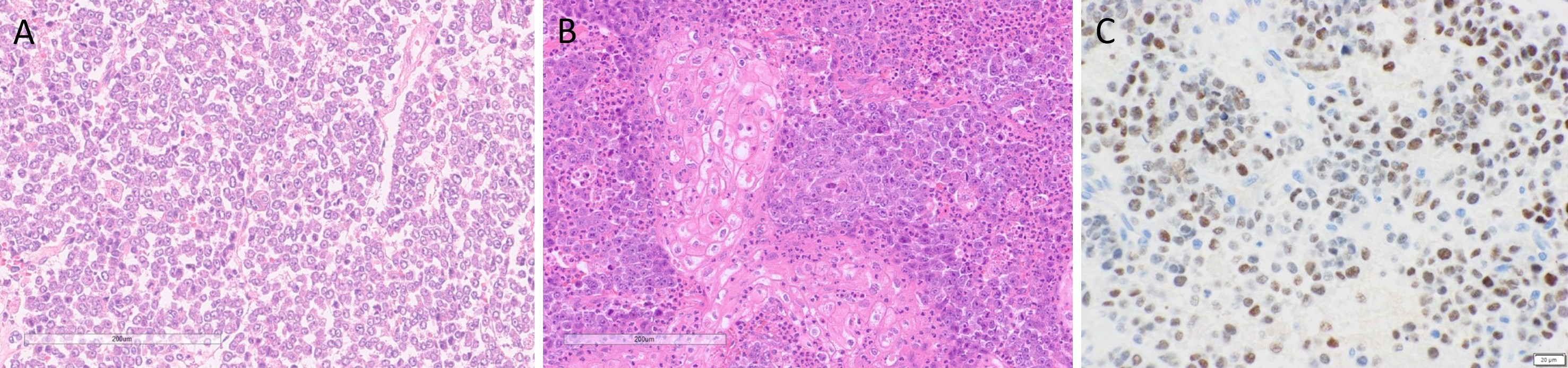
Figure 2. Histopathological features of parotid NUT carcinoma. (A) Hematoxylin and eosin (H&E) staining reveals a monotonous population of tumor cells exhibiting discohesion, nuclear enlargement, and absence of overt differentiation. (B) H&E staining highlights focal areas of keratinizing squamous differentiation, accompanied by dense neutrophilic infiltration. (C) Immunohistochemical staining for NUT demonstrates diffuse nuclear reactivity within tumor cells, confirming the diagnosis of NUT carcinoma.
Molecular Confirmation
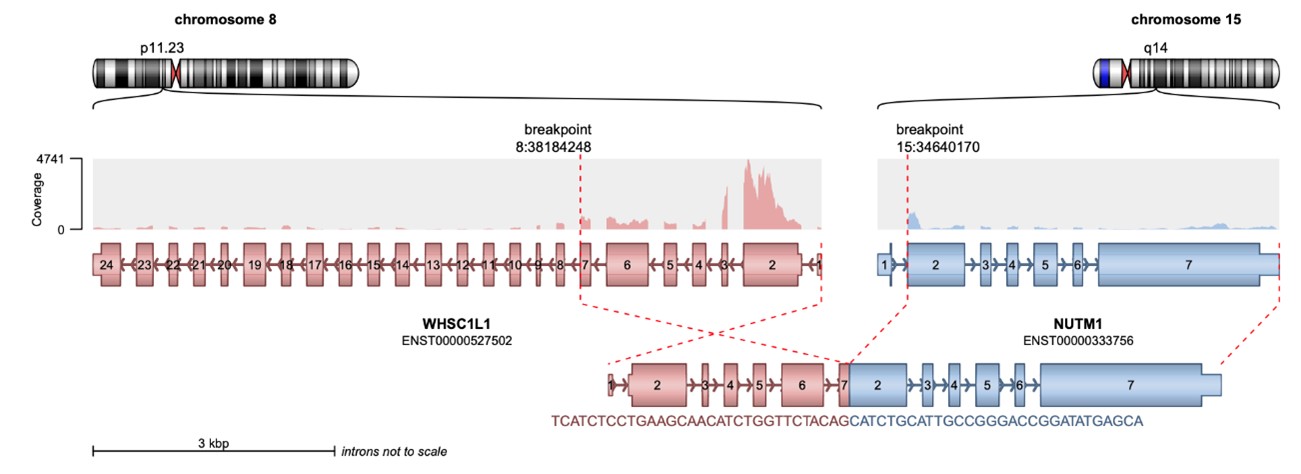
Targeted next-generation sequencing using the Illumina TruSight RNA Fusion Panel confirmed the diagnosis of NC. The analysis identified an in-frame NSD3::NUTM1 fusion, involving exons 1 through 7 of NSD3 (WHSC1L1) on chromosome 8p11.23 and exons 2 through 7 of NUTM1 on chromosome 15q14 (Figure 3). The predicted chimeric fusion protein consists of an N-terminal NSD3 segment, including the proline–tryptophan–tryptophan–proline (PWWP) histone-binding domain, fused to a nearly full-length C-terminal NUTM1 region, excluding only the six amino acids encoded by exon 1 of NUTM1. Notably, the fusion preserves the AD1 domain of NUTM1, which mediates recruitment of the histone acetyltransferase p300, contributing to megadomain formation and transcriptional activation.
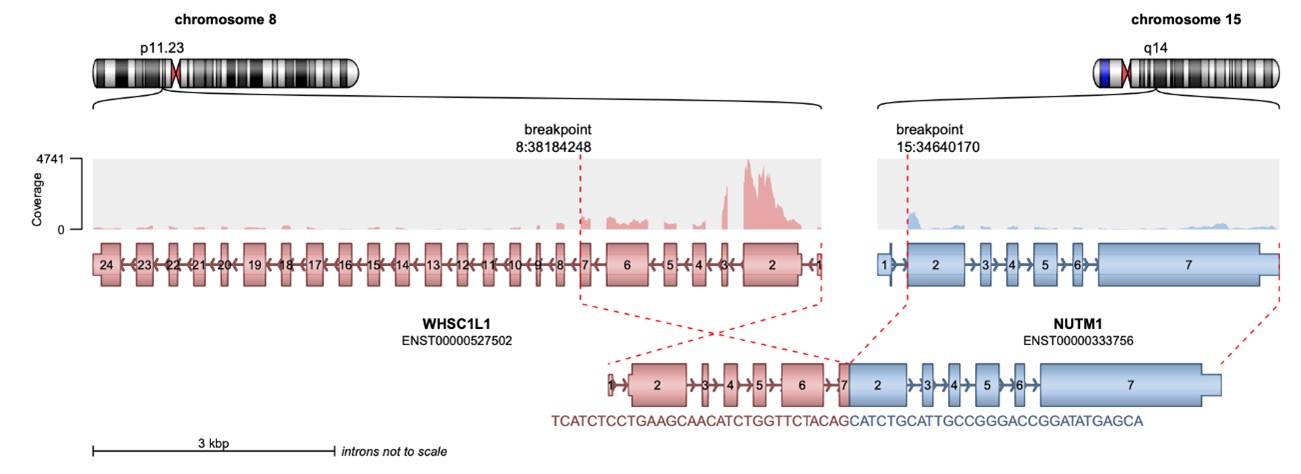
Figure 3. Schematic representation of the NSD3::NUTM1 fusion event generated using Arriba. The diagram illustrates a chromosomal rearrangement involving WHSC1L1 (also known as NSD3) located on chromosome 8p11.23 and NUTM1 on chromosome 15q14. The resulting chimeric transcript is predicted to consist of exons 1 through 7 of the 5′ partner gene NSD3 (HGNC:12767, ENST00000527502) fused in-frame to exon 2 and all subsequent exons of the 3′ partner gene NUTM1 (HGNC:29919, ENST00000333756). At the protein level, the fusion product retains the N-terminal portion of NSD3, including an intact PWWP histone-binding domain, joined to the majority of the NUTM1 protein, excluding only the six amino acids encoded by exon 1. Notably, the fusion preserves the AD1 domain of NUTM1, which mediates recruitment of the histone acetyltransferase p300, a key epigenetic regulator implicated in megadomain formation and transcriptional activation.
Postoperative Complications
Six days after surgical resection, the patient exhibited clinical deterioration marked by recurrent episodes of oxygen desaturation and apnea, prompting urgent radiologic reassessment. Repeat CT revealed extensive new metastatic lesions involving both lungs and the liver, consistent with rapidly progressive systemic dissemination. Although the postoperative pathological stage was pT4aN3bM0, indicating no distant metastases at the time of surgery, these findings reflect the highly aggressive nature of NSD3::NUTM1 fusion positive NUT carcinoma of the parotid gland. The clinical course declined rapidly, and the patient died five weeks after surgery due to complications from infection and metabolic derangement resulting from widespread metastatic disease.
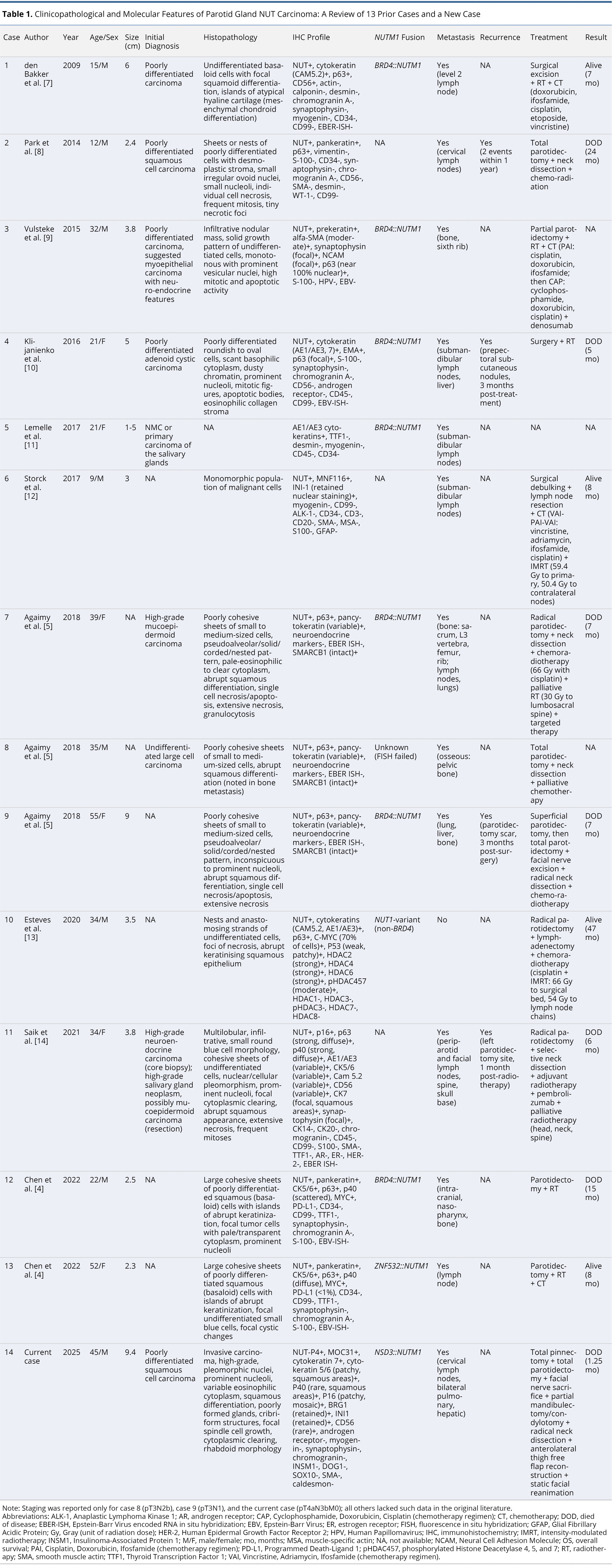
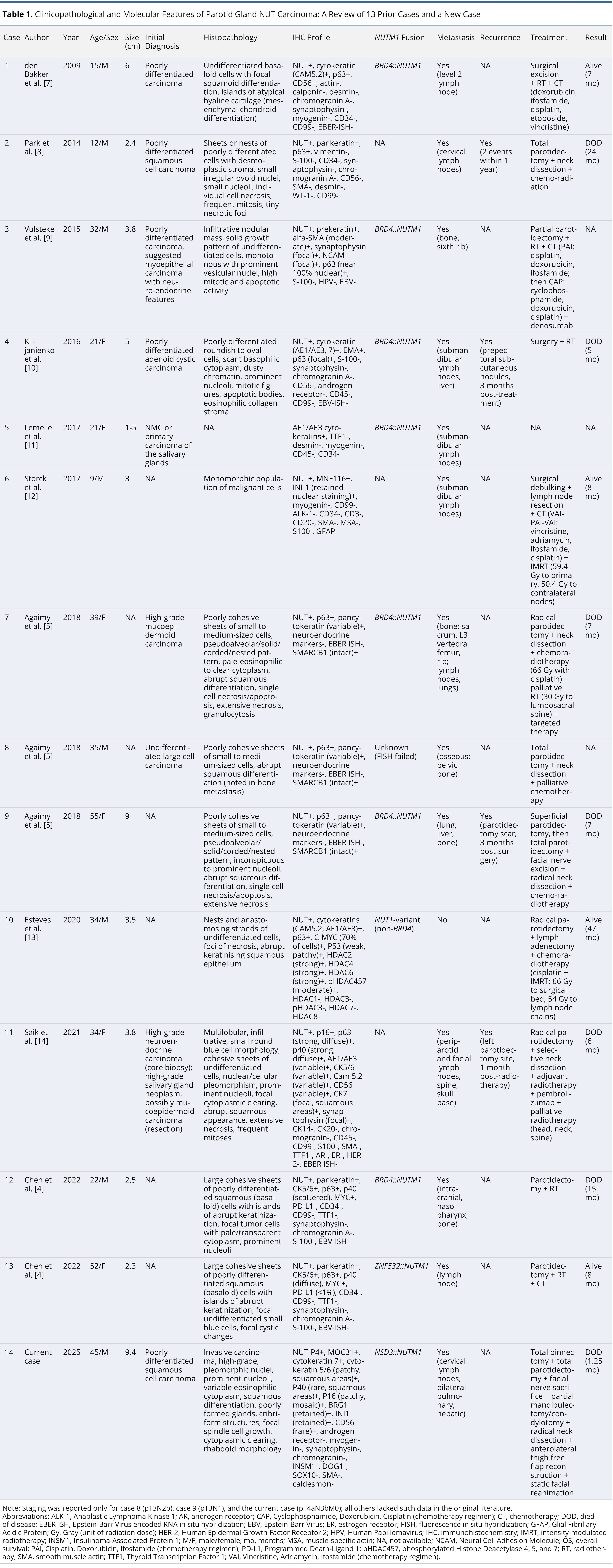
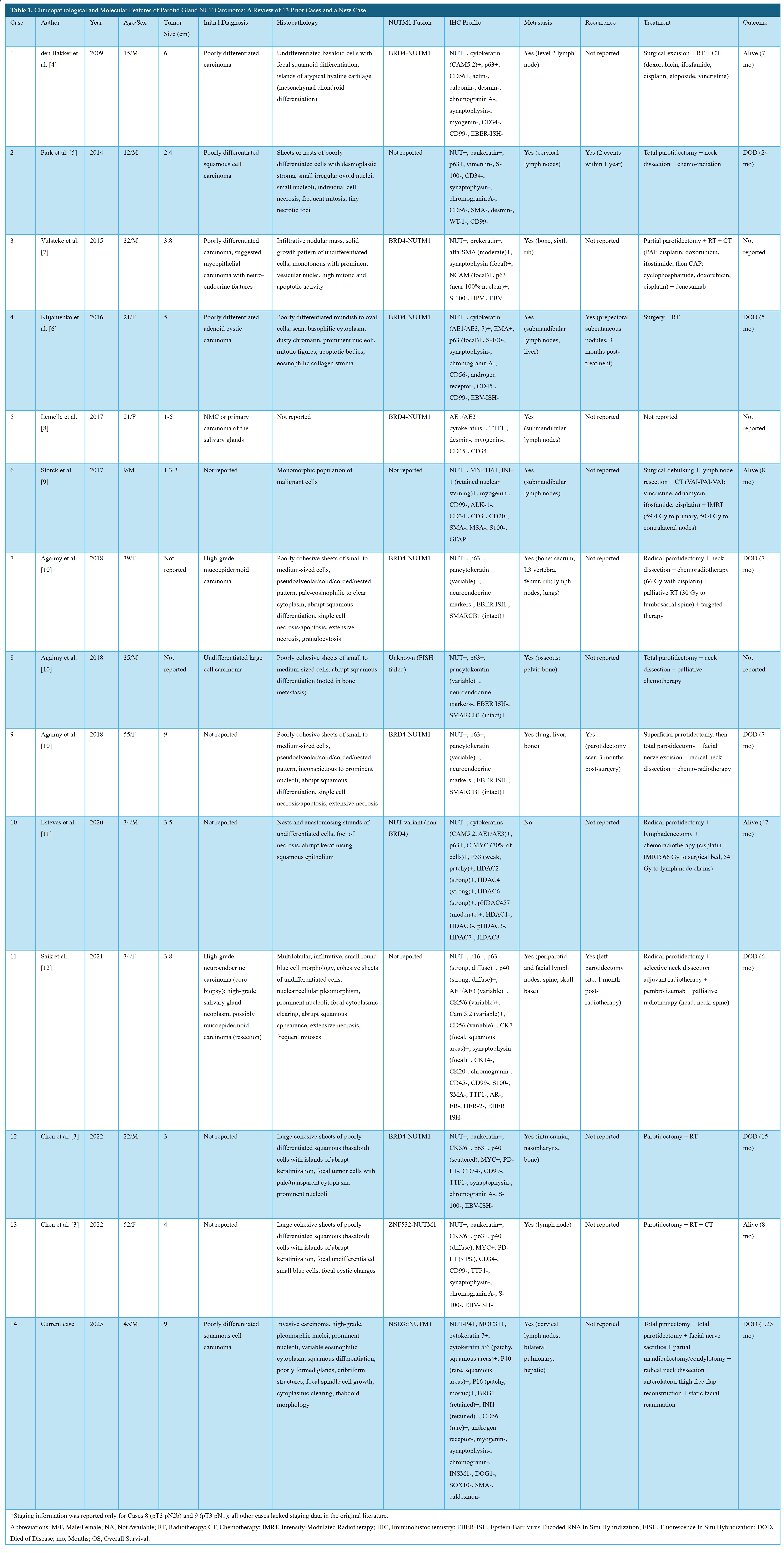
The rapid clinical progression and presence of a unique NSD3::NUTM1 fusion in the current case (case 14) underscore the highly aggressive nature and diagnostic complexity of parotid NC. Given the rarity of this tumor and the potential for initial misclassification, these observations prompted a detailed review of the literature. To contextualize the clinicopathological and molecular features of this malignancy, we examined 13 previously reported cases published between 2009 and 2022, in addition to the current case. This synthesis aimed to identify shared histopathological patterns, immunophenotypic characteristics, and fusion types, with particular emphasis on diagnostic pitfalls, metastatic behavior, and patterns of disease progression (Table 1) [4,5,7–14].
Clinicopathological Characteristics
Patient ages ranged from 9 to 55 years, with a median of 33 years. The cohort comprised eight males (57.1%) and six females (42.9%). Notably, three cases (21.4%) involved patients under the age of 20, underscoring the need to consider NC in the differential diagnosis of poorly differentiated salivary tumors in younger individuals. Tumor sizes were reported in 11 cases (cases 1 through 4, 6, and 9 through 14), ranging from 2.3 to 9.4 cm, with a median size of 3.8 cm. Size data were unavailable for cases 7 and 8, while case 5 reported only a size range (1 to 5 cm), thereby precluding inclusion in quantitative analysis. The current case (case 14) exhibited the largest tumor (9.4 cm), which demonstrated a nearly fivefold increase in volume over a two-month interval, reflecting an exceptionally aggressive growth trajectory.
Initial histopathological diagnoses were frequently nonspecific. Five cases (35.7%) were initially misclassified as either poorly differentiated carcinoma or squamous cell carcinoma, underscoring the persistent diagnostic challenges associated with this neoplastic entity. TNM staging data were available for only 3 of the 14 cases (21.4%), specifically case 8 (pT3N2b), case 9 (pT3N1), and the current case (pT4aN3bM0). This limited dataset constrains the ability to establish meaningful correlations between clinical stage and oncologic outcomes.
Histopathology
Histologically, nine of the 14 cases (64.3%) exhibited poorly differentiated carcinoma with confirmed squamous differentiation, characterized by features such as squamoid morphology, keratinization, or abrupt squamous transitions (cases 1, 7 through 14). In contrast, four cases (cases 2 through 4 and 6) were described as undifferentiated or poorly differentiated without explicit mention of squamous features, suggesting either true lack of differentiation or incomplete histologic characterization in the source reports. One case (case 5) lacked available histopathological data, precluding meaningful morphological assessment. The current case (case 14) demonstrated a particularly distinctive histological profile, exhibiting cribriform architecture and rhabdoid cytomorphology, in addition to classical NC features such as geographic necrosis and marked nuclear pleomorphism. These findings underscore the histologic heterogeneity of parotid NC and highlight the importance of recognizing subtle morphologic patterns that may prompt targeted immunohistochemical and molecular testing.
Immunohistochemistry
All 14 cases demonstrated nuclear positivity for NUT by immunohistochemistry, which remains a hallmark diagnostic feature of NC. This consistent nuclear staining supports the central role of NUTM1 rearrangements in tumor pathogenesis and affirms the utility of NUT immunohistochemistry as a frontline screening tool. Retained expression of SMARCB1/INI1 was observed across all cases, enabling distinction from SMARCB1-deficient malignancies, such as malignant rhabdoid tumors, which may exhibit overlapping morphological features. Cytokeratin markers, including CK5/6, AE1/AE3, and CK7, were universally positive (100%), reinforcing the epithelial origin of these tumors. In addition, expression of TP63 (p63) or TP40 (p40) was detected in 12 cases (85.7%), with staining patterns ranging from diffuse (>90% of tumor cells) to focal (<10%), predominantly localized to regions of squamous differentiation. For cases 5 and 6, data regarding TP63/TP40 expression were not reported, limiting full immunophenotypic comparison across the cohort.
Molecular Features
Specific NUTM1 gene fusions were identified in 10 of 14 cases (71.4%), reflecting the central role of NUTM1 rearrangements in the pathogenesis of parotid NC. Among these, seven cases (50.0%) harbored the BRD4::NUTM1 fusion, while one case (7.1%) exhibited the ZNF532::NUTM1 fusion, one case (7.1%) the NSD3::NUTM1 fusion, and one case (7.1%) an unspecified non-BRD4 variant. Fusion data were unavailable for cases 2, 6, and 11, and were inconclusive in case 8 due to failed fluorescence in situ hybridization. The novel NSD3::NUTM1 fusion identified in the current case (case 14) underscores the molecular heterogeneity observed in NUTM1-rearranged parotid carcinoma and raises the possibility of fusion-specific biological behavior. Survival duration appeared to vary by fusion type: BRD4::NUTM1 cases demonstrated survival ranging from 5 to 15 months, the ZNF532::NUTM1 case survived 8 months, the NSD3::NUTM1 case survived 1.25 months, and the non-BRD4 variant case survived 47 months. Although interpretation is limited by small sample sizes and heterogeneity in follow-up duration, these findings collectively suggest the presence of fusion-specific prognostic variability within this rare disease subset.
Metastasis
Metastatic disease was observed in 13 of 14 cases (92.9%), demonstrating the highly invasive nature of parotid NC. Lymph nodes were the most frequently involved sites, affected in 64.3% of cases, with cervical lymph node involvement specifically reported in 50.0%. In addition to nodal spread, distant metastases were also documented. Bone involvement was present in 42.9% of cases, while pulmonary and hepatic metastases were each reported in 21.4%. Case 10 was the only patient in the cohort with no evidence of metastatic spread at the time of reporting. Notably, multi-site metastases occurred in cases 7, 9, and the current case (case 14), suggesting a particularly aggressive disease course in these patients. In the current case, widespread dissemination was identified as early as six days following surgical resection, further underscoring the fulminant metastatic potential of this malignancy, even in the immediate postoperative period.
Recurrence
Tumor recurrence was clearly documented in three cases (21.4%) within one to three months following surgery (cases 4, 9, and 11), indicating a propensity for early relapse in a subset of patients. In case 2, recurrence was noted, though the precise timing was not reported, highlighting inconsistent data reporting in the literature. For the remaining 10 cases (71.4%), no information on recurrence status was available, precluding a more comprehensive evaluation of recurrence patterns or potential risk factors. The limited recurrence data further hinder exploration of correlations between molecular subtypes, treatment modalities, and relapse timing. These gaps underscore the need for standardized follow-up protocols and uniform reporting of postoperative outcomes to clarify recurrence dynamics in parotid NC.
Treatment
Surgical intervention was implemented in 13 of 14 cases (92.9%), indicating that resection remains a principal component of management for parotid NC. Radiotherapy was administered in 11 cases (78.6%) and chemotherapy in 10 cases (71.4%), reflecting the frequent use of multimodal therapeutic strategies. However, detailed information regarding the timing, dosage, or treatment objectives of these non-surgical modalities was often unavailable, limiting further comparative analysis. Treatment data for case 5 were not reported, precluding its inclusion in therapeutic outcome evaluation.
Outcome
Of the 14 cases reviewed, seven patients (50.0%) died of disease, with a reported median survival of seven months (range, 1.25 to 24 months). Four patients remained alive at the time of last follow-up, with follow-up durations ranging from 7 to 47 months. These observations reflect the marked variability in clinical trajectories associated with parotid NC. However, interpretation of outcome patterns remains limited due to the small sample size and heterogeneity in treatment strategies and reporting. Further studies are needed to clarify potential prognostic factors and optimize therapeutic decision-making.
This case report describes a 45-year-old male diagnosed with primary parotid NC harboring a rare NSD3::NUTM1 fusion, representing the first documented instance of this fusion in parotid NC. The patient initially presented with a rapidly enlarging 9.4 cm postauricular mass, initially misdiagnosed as squamous cell carcinoma. This misdiagnosis underscores the diagnostic complexity of NC due to its poorly differentiated morphology and features overlapping with squamous cell carcinoma. Despite aggressive surgical intervention, including total parotidectomy and radical neck dissection, extensive pulmonary and hepatic metastases developed within six days postoperatively, leading to the patient's death within five weeks. Our case analysis, supported by a comprehensive literature review, challenges prior reports suggesting a relatively favorable prognosis for NSD3::NUTM1 NC. Instead, it highlights significant molecular heterogeneity and aggressive metastatic behavior. Diagnostic features such as rapid tumor growth, cribriform architecture, and rhabdoid morphology underscore the importance of performing NUT immunohistochemistry and molecular analyses when NC is clinically suspected. These diagnostic strategies balance cost-effectiveness with the critical need for accurate diagnosis, which is essential for guiding potential targeted therapies, including BET inhibitors.
Detailed Analysis of the Current Case
This case of primary parotid NC harboring an NSD3::NUTM1 fusion significantly advances our understanding of the molecular and clinical aspects of this rare malignancy. Being the first documented instance of this fusion in parotid NC, it highlights the expanding spectrum of NUTM1 gene rearrangements and their contribution to aggressive tumor behavior. The present case emphasizes diagnostic and therapeutic challenges resulting from the molecular heterogeneity of NC. Consequently, detailed molecular profiling is essential to accurately differentiate NC from other salivary gland malignancies. This analysis further investigates the biological implications of the NSD3::NUTM1 fusion, clinical progression, diagnostic complexities, prognostic variations, and potential therapeutic strategies, providing valuable direction for future research and clinical management.
Molecular characteristics
NC is defined by chromosomal rearrangements of the NUTM1 gene, most commonly fused with BET family genes such as BRD4 or BRD3 [15]. However, this case is distinguished by the presence of an NSD3::NUTM1 fusion, a variant that has been infrequently reported in NCs [6]. To our knowledge, this is the first reported case of a primary parotid gland NC harboring this specific fusion. This finding expands our understanding of the molecular heterogeneity of NCs and highlights the importance of comprehensive molecular testing in the diagnosis of poorly differentiated carcinomas.
Fusion biology and epigenetic implications
The NSD3::NUTM1 fusion is particularly noteworthy because of its rarity and significant biological implications. NSD3 is a histone methyltransferase within the Nuclear SET Domain-containing (NSD) protein family [16]. It binds specifically to the extraterminal domain of BET proteins, suggesting that the NSD3::NUTM1 fusion oncogene shares functional similarities with the well-characterized BRD4::NUTM1 oncogene [17]. This fusion disrupts normal cellular differentiation pathways, leading to sustained proliferation of poorly differentiated tumor cells.
Clinical behavior
From a clinical perspective, this case aligns with the known aggressive nature of NC. Despite thorough preoperative imaging, including CT, PET, and MRI, no distant metastases were initially detected. Nevertheless, the patient experienced rapid disease progression, developing extensive metastases shortly after surgical intervention and ultimately succumbing to the disease within five weeks. This rapid clinical deterioration and early postoperative metastatic spread reflect the highly aggressive biological behavior commonly associated with NC. These findings are supported by existing literature; for example, Agaimy et al. analyzed 10 cases of salivary gland NC, reporting that most patients developed widespread metastases soon after surgery, with seven patients (70%) dying between 1 and 24 months postoperatively (median survival of 5.5 months) [5].
Diagnostic challenges
The absence of detectable distant metastases on comprehensive preoperative imaging in this case highlights a diagnostic limitation. This issue reflects not the inadequacy of the imaging modalities themselves, but the tumor’s intrinsic biological aggressiveness and its capacity for rapid systemic dissemination. Although CT, MRI, and PET provide high-resolution anatomical and functional evaluations, these modalities are inherently limited in detecting micrometastatic disease. This limitation is particularly pronounced in tumors characterized by accelerated proliferative kinetics.
In this case, the initial impression of radiologic stability prior to surgery was misleading. Within six days, extensive pulmonary and hepatic metastases emerged. This progression exemplifies a critical diagnostic gap. The gap stems from a temporal discrepancy between the timing of imaging and the real-time biological dynamics of an epigenetically dysregulated malignancy such as NC.
Accurate diagnosis is further complicated by the histopathological ambiguity of NC. The tumor often exhibits poorly differentiated morphology with abrupt squamous features. Such patterns can result in initial misclassification as squamous cell carcinoma or other high-grade salivary gland neoplasms. In this case, the diagnostic challenge was amplified by the rarity of parotid NC and its overlap with more prevalent malignancies.
Routine application of NUT immunohistochemistry in all undifferentiated parotid tumors is not feasible due to cost and resource constraints. However, in the presence of specific clinical and histological indicators, targeted testing becomes essential. These indicators include rapid tumor growth, unusually young patient age for salivary gland malignancy, and high-grade morphology with squamous differentiation. In such scenarios, immunohistochemical detection of NUT protein should be promptly performed. This should be followed by molecular confirmation using next-generation sequencing or fluorescence in situ hybridization.
A selective, criteria-based approach to diagnostic testing ensures efficient resource utilization. At the same time, it enables timely and accurate identification of NC. Early diagnosis is crucial for prognostic assessment, therapeutic planning, and consideration of targeted treatments such as BET inhibitors.
Prognostic discrepancies
While NC typically carries a poor prognosis, recent analyses suggest survival outcomes may differ based on anatomical site and specific fusion type. A risk model by Chau et al. indicated patients with non-thoracic primary tumors harboring BRD3::NUT or NSD3::NUT fusions had significantly longer median survival durations, approximately 36.5 months [2]. However, the aggressive clinical progression observed in the current case, marked by rapid metastasis and patient death within two months postoperatively despite having an NSD3::NUTM1 fusion originating in the parotid gland, notably diverges from these findings. This discrepancy highlights limitations within existing prognostic frameworks and underscores the possibility of substantial biological variability even among rare fusion subtypes such as NSD3::NUTM1, for which data are limited (only seven cases reported by Chau et al. [2]). Consequently, this case emphasizes the urgent need for refined prognostic stratification in future studies to more accurately predict clinical outcomes in this aggressive malignancy.
Survival analysis
An integrated review of previously reported parotid NC cases, along with the current case (Table 1), shows a median survival of approximately seven months. This finding highlights the inherently aggressive nature of parotid NC. The fulminant progression and rapid fatality observed in our patient further reinforce this observation. The patient survived only 1.25 months after surgery. This outcome contrasts sharply with the 36.5-month median survival reported for NSD3::NUTM1 fusion-positive non-thoracic carcinomas, most of which arise in midline head and neck sites [2]. Although interpretation is limited by the inclusion of only one parotid case with NSD3::NUTM1 fusion, the marked survival disparity suggests a potentially more aggressive clinical phenotype. This difference raises the possibility that other biological factors may influence disease behavior. These may include differences in the tumor microenvironment, variability in fusion breakpoint architecture, or the presence of additional genetic alterations.
Therapeutic implications
The identification of the rare NSD3::NUTM1 fusion presents potential therapeutic implications. Recent interest in small molecule BET inhibitors stems from their effectiveness in targeting BRD4-NUTM1 oncoproteins associated with NC [18]. Although clinical data specifically evaluating BET inhibitors in NSD3::NUTM1 fusion tumors remain limited, experimental studies have shown that NSD3-NUT fusion proteins maintain the proliferative and undifferentiated state of NC cells. These fusion proteins mechanistically resemble the BRD4-NUT complexes known to drive tumor growth. Preliminary studies indicate BET inhibitors, such as JQ1, can disrupt these mechanisms by inducing cellular differentiation and reducing proliferation in models expressing the NSD3::NUT fusion [19]. These findings highlight BET inhibition as a potentially promising therapeutic approach for tumors harboring the NSD3::NUTM1 fusion, although further preclinical and clinical investigations are necessary to validate efficacy and clinical applicability.
Literature and Case Comparison
The aggressive clinical progression and identification of the novel NSD3::NUTM1 fusion in this case highlight the diagnostic and therapeutic complexities associated with parotid NC. Consequently, we conducted a comparative analysis with previously reported cases. This review compiles data from 13 cases documented between 2009 and 2022, together with the current case, to comprehensively detail their clinical, histopathological, and molecular characteristics. The analysis aims to enhance understanding of diagnostic challenges and prognostic factors associated with parotid NC.
Aggressiveness and molecular heterogeneity
Parotid NC is recognized for its highly aggressive clinical behavior, characterized by rapid tumor growth, extensive metastasis, and poor overall prognosis, posing substantial clinical management challenges. The central pathogenic feature of NC involves NUTM1 gene fusions, which alter the chromatin landscape via epigenetic dysregulation, resulting in aberrant activation of genes related to cell proliferation. Specifically, the BRD4::NUTM1 fusion recruits histone acetyltransferase EP300 to create hyperacetylated chromatin domains (megadomains), significantly enhancing transcriptional activity of key oncogenes such as MYC and TP63 [1]. In this review, elevated MYC expression was consistently noted in cases with the BRD4::NUTM1 fusion (e.g., cases 10, 12, 13), underscoring its essential role in tumor growth and differentiation blockade [20]. Therapeutic targeting of MYC or the upstream BRD4::NUTM1 complex, potentially through BET inhibitors, holds promise for tumors harboring this fusion.
However, regulatory mechanisms governing MYC expression in non-BRD4 fusions, such as NSD3::NUTM1, require further exploration. In contrast, the NSD3::NUTM1 fusion may predominantly modulate H3K36 methylation, suppressing differentiation-related genes and promoting an undifferentiated tumor phenotype [17]. Other rare fusion variants, like ZNF532::NUTM1, remain inadequately characterized; preliminary data suggest they induce similar chromatin alterations associated with aggressive tumor behavior [17]. The broad spectrum of fusion variants identified highlights the significant molecular diversity in parotid NC, distinct from previously described cases dominated by BRD4::NUTM1. This heterogeneity potentially influences not only the underlying tumor biology but also therapeutic responsiveness, including BET inhibitor sensitivity in NSD3::NUTM1 cases and potential survival differences among various fusion subtypes. Future research should focus on elucidating fusion-specific epigenetic mechanisms to facilitate the development of targeted therapeutic strategies tailored to distinct molecular profiles.
Histopathology and diagnostic features
The histopathologic characteristics of parotid NC exhibit considerable heterogeneity. Most reported cases demonstrate features consistent with poorly differentiated carcinoma, frequently displaying squamous differentiation, including squamoid, keratinizing, or abrupt squamous morphologies. Positive NUT immunohistochemistry is a defining diagnostic hallmark of NUT carcinoma. When accompanied by retained SMARCB1/INI1 expression, it reliably distinguishes NC from SMARCB1-deficient malignancies, including malignant rhabdoid tumors. Immunohistochemical positivity for cytokeratins (CK5/6, AE1/AE3, CK7) and p63/p40 further supports an origin from parotid ductal squamous epithelium, consistent with the observed aggressive clinical behavior [4]. The histologic implications of other fusion variants, notably ZNF532::NUTM1, remain insufficiently characterized but likely involve comparable epigenetic alterations [17]. These findings underscore the diagnostic importance of NUT immunohistochemistry and highlight the necessity of further research into fusion-specific morphological features, which is critical for precise differential diagnosis and molecular subtyping.
Metastatic patterns and invasiveness
Parotid NC demonstrates a significant propensity for metastasis, commonly spreading to cervical lymph nodes, bone, lung, and liver, emphasizing its aggressive nature. Particularly rapid metastatic progression has been observed in cases with the NSD3::NUTM1 fusion. The rare occurrence of cases without detectable metastasis may be attributed to early diagnosis or specific molecular subtype characteristics, though these possibilities require further investigation. The rapid metastatic pattern underscores the critical need for prompt initiation of systemic therapies. Future studies should aim to elucidate the molecular mechanisms underlying fusion-specific metastatic behaviors and identify reliable biomarkers for predicting metastatic risk, thereby improving the efficacy of therapeutic interventions.
Study Limitations
This review included an analysis of 13 previously reported cases and one newly documented case of parotid NC. A significant limitation of the current study is the substantial heterogeneity and incompleteness of available case data, which restricts the depth of prognostic insights. Critical details such as TNM staging, tumor size, recurrence status, and molecular fusion types were often unavailable, thereby complicating accurate evaluations of survival and recurrence risks. Furthermore, variability in treatment approaches, including surgery, radiotherapy, and chemotherapy, along with inconsistent reporting standards, further challenge the robustness of comparative analyses. Since most data originate from individual case reports or small case series, potential selection biases may impact the broader applicability of these findings.
Future Research
Future research should emphasize comprehensive molecular sequencing to systematically explore epigenetic mechanisms and treatment responses specific to non-BRD4::NUTM1 fusion types, including NSD3::NUTM1 and ZNF532::NUTM1. Prospective clinical trials are essential for enhancing median survival outcomes and elucidating molecular underpinnings of survival variability across different fusion variants. Clinically, given the frequent misdiagnosis and rapid progression associated with parotid NC, NUT immunohistochemistry should be selectively applied in cases with suggestive clinical or histopathological features. Additionally, integrating advanced molecular diagnostic techniques such as next-generation sequencing or fluorescence in situ hybridization to detect NUTM1 fusions can facilitate early diagnosis and personalized treatment strategies. Considering the aggressive metastatic potential of parotid NC, future studies should prioritize identifying predictive biomarkers and evaluating combination therapeutic strategies to improve clinical management and patient survival.
This report presents the first documented case of parotid NC harboring an NSD3::NUTM1 fusion, significantly expanding the molecular understanding of this rare and highly aggressive tumor type. A comprehensive analysis of 14 cases, including 13 previously reported and the current case, reveals a metastatic rate of 92.9%, frequent initial misdiagnosis as poorly differentiated carcinoma, and a median overall survival of approximately seven months. These findings underscore the substantial molecular heterogeneity and diagnostic difficulties inherent to NC. Considering these complexities, NC should consistently be included in the differential diagnosis of poorly differentiated malignancies of the parotid gland. Accurate diagnosis is critically dependent on NUT protein detection via immunohistochemistry and confirmation through next-generation sequencing. Additional research focusing on the molecular mechanisms of NC, particularly concerning non-BRD4::NUTM1 fusion variants and related epigenetic pathways, is imperative for identifying clinically relevant biomarkers. This study emphasizes the importance of comprehensive molecular profiling to enhance diagnostic accuracy and improve patient outcomes. Additionally, it highlights the pressing necessity for developing targeted therapeutic approaches specific to distinct fusion variants.
Received date: April 02, 2025
Accepted date: May 10, 2025
Published date: May 19, 2025
The manuscript has not been presented or discussed at any scientific meetings, conferences, or seminars related to the topic of the research.
The study adheres to the ethical principles outlined in the 1964 Helsinki Declaration and its subsequent revisions, or other equivalent ethical standards that may be applicable. These ethical standards govern the use of human subjects in research and ensure that the study is conducted in an ethical and responsible manner. The researchers have taken extensive care to ensure that the study complies with all ethical standards and guidelines to protect the well-being and privacy of the participants.
The author(s) of this research wish to declare that the study was conducted without the support of any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) conducted the study solely with their own resources, without any external financial assistance. The lack of financial support from external sources does not in any way impact the integrity or quality of the research presented in this article. The author(s) have ensured that the study was conducted according to the highest ethical and scientific standards.
In accordance with the ethical standards set forth by the SciTeMed publishing group for the publication of high-quality scientific research, the author(s) of this article declare that there are no financial or other conflicts of interest that could potentially impact the integrity of the research presented. Additionally, the author(s) affirm that this work is solely the intellectual property of the author(s), and no other individuals or entities have substantially contributed to its content or findings.
It is imperative to acknowledge that the opinions and statements articulated in this article are the exclusive responsibility of the author(s), and do not necessarily reflect the views or opinions of their affiliated institutions, the publishing house, editors, or other reviewers. Furthermore, the publisher does not endorse or guarantee the accuracy of any statements made by the manufacturer(s) or author(s). These disclaimers emphasize the importance of respecting the author(s)' autonomy and the ability to express their own opinions regarding the subject matter, as well as those readers should exercise their own discretion in understanding the information provided. The position of the author(s) as well as their level of expertise in the subject area must be discerned, while also exercising critical thinking skills to arrive at an independent conclusion. As such, it is essential to approach the information in this article with an open mind and a discerning outlook.
© 2025 The Author(s). The article presented here is openly accessible under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). This license grants the right for the material to be used, distributed, and reproduced in any way by anyone, provided that the original author(s), copyright holder(s), and the journal of publication are properly credited and cited as the source of the material. We follow accepted academic practices to ensure that proper credit is given to the original author(s) and the copyright holder(s), and that the original publication in this journal is cited accurately. Any use, distribution, or reproduction of the material must be consistent with the terms and conditions of the CC-BY license, and must not be compiled, distributed, or reproduced in a manner that is inconsistent with these terms and conditions. We encourage the use and dissemination of this material in a manner that respects and acknowledges the intellectual property rights of the original author(s) and copyright holder(s), and the importance of proper citation and attribution in academic publishing.
This case reports a 40-year-old male patient with right sinonasal teratocarcinosarcoma. It is a rare malignant lesion with extremely aggressive and commonly recurrent nature. The average survival period is less than two years.
The author presents a case of BCC developed on an active lesion of mucocutaneous leishmaniasis as one of many cases of skin cancer frequently seen at the Regional Leishmaniasis Control Center (RLCC) clinics in Yemen.
Dysphagia is an important consequence of cancer treatment and has overarching implications on quality of life. Using the FOIS, we demonstrated that swallowing function may be worse in the long term in patients with OPSCC undergoing triple therapy, although this finding did not reach statistical significance. This study emphasizes the importance of diligent selection in patients undergoing TORS to avoid poor functional swallowing outcomes, particularly in those that may need adjuvant chemoradiation therapy. A study with a larger sample size may determine the significance of these trends.
Psammoma bodies are a characteristic of papillary thyroid carcinoma. The prognostic value of thyroid psammoma bodies, however, has not been clarified. The authors conducted this study to ascertain whether non-tumoral psammoma bodies are associated with undesirable histological findings. A study of 584 cases discovered that non-tumoral psammoma bodies were associated with extensive lymphatic spread and more aggressive tumor growth in classic type papillary thyroid carcinoma. As recommended by the authors, it is crucial to mention the presence of psammoma bodies in the pathology report.
The incidence of high-grade sinonasal adenocarcinomas of non-intestinal origin is extremely rare. In this case report, the authors present a very rare case of high-grade non-intestinal sinonasal adenocarcinoma presenting with a challenging diagnosis. Because of its significantly different prognosis, this study provides a detailed explanation of how the authors differentiate it from other sinonasal tumors. In addition, they describe how a radical endoscopic resection was applied in order to achieve a total excision.
This article holds critical relevance for healthcare professionals, particularly in the fields of microsurgery, oncology, and vascular medicine. It thoroughly examines the diagnostic challenges faced in distinguishing between recurrent lymphedema and deep vein thrombosis in elderly cancer patients following lymphovenous anastomosis surgery. It highlights the significant risk of misdiagnosing deep vein thrombosis as lymphedema, a mistake that can delay critical treatment due to their clinical similarities. The case study of a 79-year-old patient emphasizes the importance of a comprehensive reassessment, considering the patient's entire medical history, including the effects of cancer treatments like immunotherapy. The article stresses the need for a holistic approach to patient management and the utilization of advanced diagnostic tools for accurate diagnosis and treatment. It is essential reading for its insights into the complex dynamics of postoperative care and the critical importance of accurate diagnosis in treating elderly cancer patients effectively.
This article presents a unique surgical case of a 75-year-old Chinese woman with a parotid lesion, highlighting the unpredictability of human anatomy. It underscores the critical need for flexibility in surgical techniques, especially when faced with deviations in the facial nerve's pathway. Traditional intraoperative landmarks proved unreliable due to significant anatomical displacement, prompting a shift from the conventional anterograde to a retrograde dissection approach. This case study serves as a crucial reminder of the importance of tailor-made strategies in surgery, offering invaluable insights for medical professionals in navigating complex cases and ensuring patient safety.
This study reports the first case of a highly aggressive primary parotid NUT carcinoma with an NSD3::NUTM1 fusion, challenging existing assumptions about the clinical behavior of this rare tumor. Prior studies have indicated that NSD3::NUTM1 fusions in non-thoracic NUT carcinomas, primarily in midline head and neck or bone/soft tissue sites, are associated with extended survival (median 36.5 months; Chau et al., 2019), though no data exist for salivary gland tumors. In contrast, the patient in this report exhibited rapid disease progression, with widespread metastases developing soon after diagnosis and death occurring within 2 months after surgery. This report provides new evidence suggesting that primary parotid NSD3::NUTM1 NUT carcinomas may exhibit a more aggressive clinical trajectory than non-thoracic NSD3::NUTM1 NUT carcinomas, primarily in midline head and neck sites (Chau et al., 2019), although direct comparisons are limited by the absence of prior parotid NSD3::NUTM1 data. The observed discordance between molecular characteristics and clinical behavior implies the potential influence of additional factors, including tumor microenvironmental conditions, specific fusion breakpoints, or the presence of cooperating genetic alterations. This case thus opens avenues for future research focused on elucidating the complex biological underpinnings of NUT carcinoma aggressiveness. Importantly, the findings reinforce the critical role of comprehensive molecular diagnostics in the management of rare malignancies. Advanced molecular testing not only enables accurate diagnosis and informs immediate treatment strategies but also predicts prognosis, facilitates access to precision clinical trials, and contributes valuable data to enhance future risk prediction models. As additional cases are identified and systematically analyzed, there is hope for the development of more accurate prognostic frameworks to guide clinical decision-making and improve patient outcomes. This study, through integrated clinical and molecular analysis, fills a critical knowledge gap and prompts reevaluation of NUT carcinoma risk classification. In precision oncology, the dynamic interplay of molecular alterations and clinical behavior necessitates ongoing updates to diagnostic and therapeutic strategies to enhance outcomes for rare, aggressive cancers. Given its significant academic and clinical impact, this study is well-suited for publication with minor revisions.
The manuscript presents a case study of primary parotid NUT carcinoma with an NSD3::NUTM1 fusion, addressing a critical gap in understanding the clinical behavior and therapeutic management of this rare fusion variant. While the case provides valuable clinical and molecular insights, revisions are needed to improve logical flow and scientific rigor. In particular, greater clarity is required in the discussion of BET inhibitor susceptibility.
This study reports a rare case of primary parotid NUT carcinoma harbouring an NSD3::NUTM1 fusion, contributing valuable insights into the behaviour of fusion-driven tumours beyond midline structures. Comprehensive preoperative imaging, including computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI), revealed no evidence of distant metastases. However, the patient developed rapid, widespread metastatic disease shortly after surgery, succumbing to the illness within five weeks. This aggressive clinical course underscores the distinctive biological characteristics of NUT carcinoma, aligning with prior reports of post-surgical dissemination and poor prognosis. By meticulously documenting the abrupt postoperative progression and confirming its independence from diagnostic oversight, the authors enhance the clinical relevance of their findings. To further strengthen the impact of this work, additional details on imaging-based evidence of tumour growth dynamics would be advantageous.
The authors present a clinically instructive case of primary parotid NUT carcinoma with an NSD3::NUTM1 fusion, highlighting the biological heterogeneity and prognostic complexity associated with this aggressive malignancy. While previous large-scale analyses have associated NSD3::NUTM1 fusion and non-thoracic primary sites with relatively favorable outcomes, the markedly rapid disease progression observed in this patient underscores the limitations of current risk models and highlights the need for more granular molecular stratification. By critically juxtaposing the present case against established prognostic frameworks, the manuscript compellingly argues that fusion type and anatomical site alone may be insufficient to predict clinical behavior. Furthermore, the discussion appropriately contextualizes the potential therapeutic relevance of BET inhibitors, exercising commendable scientific caution by emphasizing that their efficacy in NSD3::NUTM1-positive tumors remains hypothetical and requires empirical validation. Through its careful synthesis of clinical observation and critical analysis, this study meaningfully advances the evolving understanding of NUT carcinoma and its prognostic determinants. With modest edits, the article could be considered for publication.
In Figure 2, we observed that the label "A" in panel A overlaps with the scale bar, obscuring it and hindering a clear view of the scale and the full pathological context of the image. To enhance clarity and ensure accurate data presentation, we kindly request the original images without the "A" or "B" labels. This allows for better observation of the pathological features.
RESPONSEWe have added the original images.
Weerakkody D, Mitchell C, Newman M, McEvoy C, Gan T, Zhang M, Ng S. NSD3::NUTM1 fusion in primary parotid NUT carcinoma: First report and comprehensive literature review. Arch Otorhinolaryngol Head Neck Surg 2025;9(1):2. https://doi.org/10.24983/scitemed.aohns.2025.00196