Inflammatory myofibroblastic tumor (IMT) is a rare, mesenchymal low-grade neoplasm characterized by benign histology but has the potential of showing aggressive clinical behavior with local invasiveness and recurrence. It primarily occurs in children and young adults in the lung and abdominopelvic regions, but there are reports of oral cavity IMTs. In this report, the authors present a case of a neonate born with an enlarging tongue mass whose biopsy revealed IMT with anaplastic lymphoma kinase (ALK) positivity. Treatment included tumor debulking and an ALK inhibitor, crizotinib, which resulted in complete remission. To our knowledge, this is the only reported case of oral cavity IMT with ALK positivity in a neonate.
Inflammatory myofibroblastic tumor (IMT) is a rare, mesenchymal neoplasm primarily occurring in the lung and abdominopelvic regions in children and young adults with a prevalence ranging from 0.04-0.7% [1-3]. IMT presents as a painless, enlarging mass and may show invasiveness and local recurrence but is rarely metastatic [4].
Histologic characteristics include a mixture of inflammatory and myofibroblastic spindle cells with hypocellular fibrous patterns that can be well-circumscribed to ill-defined on imaging [1,3]. Surgical intervention have been mostly noted as the mainstay treatment for IMTs, though chemotherapy agents and anti-inflammatory therapies such as steroidal and non-steroidal anti-inflammatory drugs have been reported, as well [5].
To date, there are published cases of IMT involving the head and neck, including the oral cavity, but to our knowledge, there are no reported studies of inflammatory myofibroblastic tumor with anaplastic lymphoma kinase (ALK) positivity of the tongue in a neonate [2,4,6-16].
A 0-day-old neonate, born without complication at 41 weeks, presented as a transfer from an outside facility to Children’s Mercy Hospital (CMH) in Kansas City, Missouri for further evaluation and management of a large, bleeding tongue mass. Of note, there was reported comprehensive antenatal care without complication or detection of the tongue mass on three prenatal ultrasounds. At birth, the tongue mass was noted. The patient did not have significant airway obstruction with APGARs 8 and 9 at one and five minutes of life, respectively. On physical exam, there was a large, firm, oozing tongue mass with transition from normal appearing tongue mucosa to necrotic and atypical tongue mucosa roughly halfway posterior on the lingual surface (Figure 1). The patient’s airway was widely patent on flexible laryngoscopic exam, and she was saturating well on room air.
The patient then underwent transnasal intubation in the operating room and magnetic resonance imaging (MRI). MRI face with and without contrast showed a large mass that filled and distorted the normal anatomy of the oral cavity and floor of mouth with extent inferiorly and laterally to the level of the mandible without bony extension and superiorly to the level of the hard palate and posteroinferiorly to the vallecula, measuring 3.4 x 5.5 x 3.1 cm. The mass was isointense to soft tissue on T1 and heterogeneously hyperintense on T2 with absence of vascular flow voids (Figure 2). MRI brain was noted to be normal.
Biopsy of the tongue mass, tracheotomy, and gastrostomy tube placement occurred. Pathology, which was analyzed by CMH and an outside facility, was consistent with a congenital inflammatory myofibroblastic tumor with ALK, S100-protein, smooth muscle actin (SMA), desmin, and p53 positivity.
Traditional therapy would be complete resection, but in this patient would require subtotal glossectomy. Due to the high morbidity with this procedure, we received compassionate approval for targeted therapy with an ALK inhibitor, crizotinib. Prior to initiation of therapy, due to the necrotic nature of the tumor, dubulking was performed. Despite initial growth of the tumor, after twelve months of receiving crizotinib, there was good response with noticeable shrinkage of the tumor (Figure 3A). The patient did have prolongation of her QTc interval, though a normal baseline echocardiogram, which can be a side effect of crizotinib. She underwent serial electrocardiograms (EKGs) during treatment to monitor her QTc interval, which normalized after cessation of crizotinib. The patient was last seen at 18 months old , off of therapy for eight months without signs or symptoms of recurrence with no detectable tumor by MRI imaging (Figure 2B). She has been decannulated with removal of her gastrostomy tube without difficulties talking, swallowing, or breathing (Figure 3B).
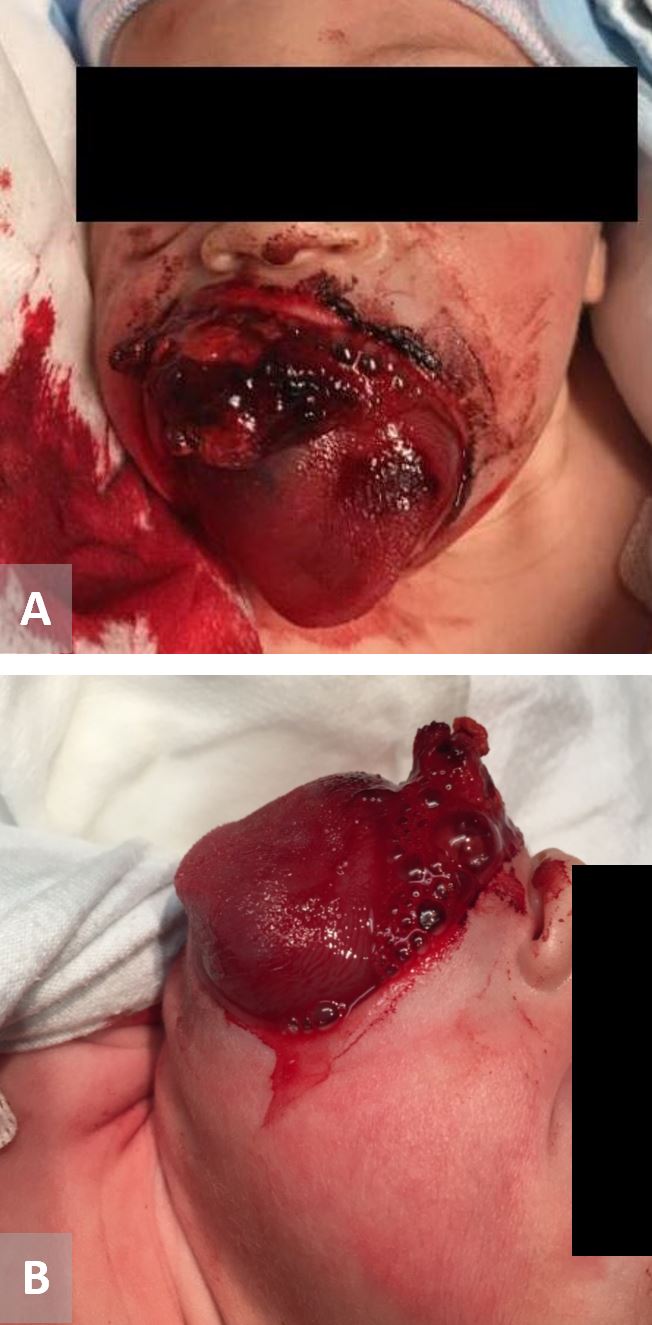
Figure 1. 24-hour neonate with a several centimeter tongue mass with hemorrhage. (A) Frontal view. (B) Lateral view.
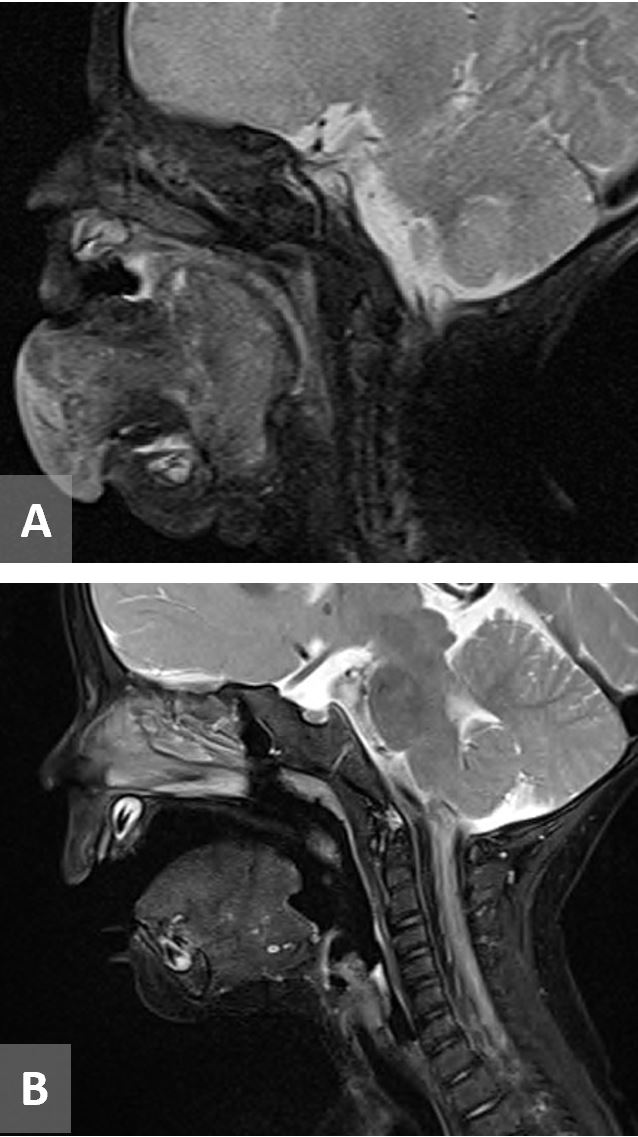
Figure 2. (A) MRI T2 sequence showing a large tongue mass that is heterogeneously hyperintense without vascular flow voids measuring 3.4 x 5.5 x 3.1 cm. (B) MRI T2 sequence 5 months post-treatment. MRI, magnetic resonance imaging.
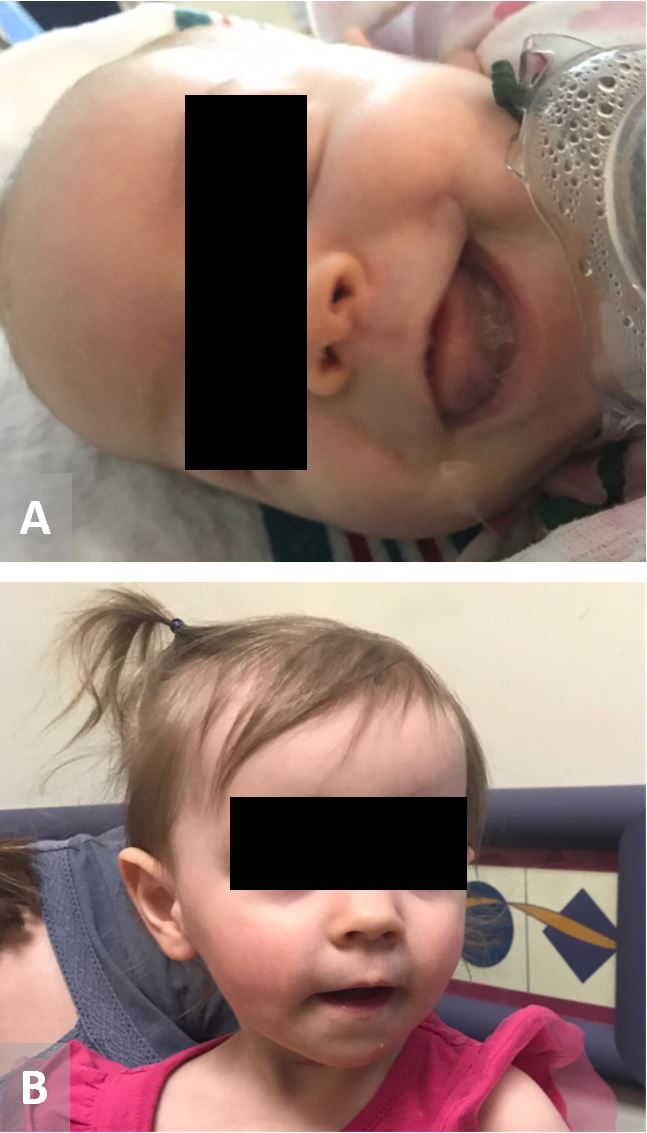
Figure 3. Shrinkage of tongue mass after initiation of chemotherapeutic agent, crizotinib. (A) At 2 months of age. (B) At 18 months of age.
Overview
Inflammatory myofibroblastic tumor is an overall rare, mesenchymal neoplasm that occurs primarily in the lung and abdominopelvic regions, with only 14-18% of the extrapulmonary IMTs occurring in the head and neck region [5]. Of that small subset of head and neck occurrences, the orbits and upper airways are most common [5]. Our study adds to the literature in that it is a tongue IMT, positive for ALK, in a neonate.
Pathological, Histological, and Imaging Features
Extrapulmonary cases of inflammatory myofibroblastic tumor show a combination of spindled fibroblastic and myofibroblastic cells with inflammatory infiltrate of lymphocytes, eosinophils and plasma cells [17]. The pathology in our patient showed relatively monotonous sheets of cells with ovoid to spindle-shaped nuclei, indistinct pale-gray cytoplasm with a delicate slightly vacuolated chromatin pattern (Figure 4). Mitotic figures and foci of necrosis visible. Other features are prominent hemangiopericytomatous vasculature with lymphocytic infiltrate and focal collections of osteoclastic giant cells. Fluorescence in situ hybridization (FISH) was positive for ALK in our patient. Positivity for the ALK gene has been reported in approximately 50% of IMTs [1,18]. The diagnosis of IMT requires histological and immunohistochemical staining to confirm diagnosis [8]. In this case, ALK, S-100 protein, SMA, desmin and p53 was positive. In a study observing immunohistochemical aspects of IMT, it showed vimentin was present in 100% of the cases (30/30) then in descending order SMA at 70% (21/30), desmin and ALK at 27% (8/30), and cytokeratin (CK) at 13% (4/30) [17]. Another study confirmed that most commonly SMA and vimentin are found to be positive to diagnose IMT [19].
Imaging features are variable depending where they are found in the body. Commonly, one will see an ill-defined soft tissue mass with heterogenous enhancement with or without invasive features on computed tomographic (CT) or MRI [19].
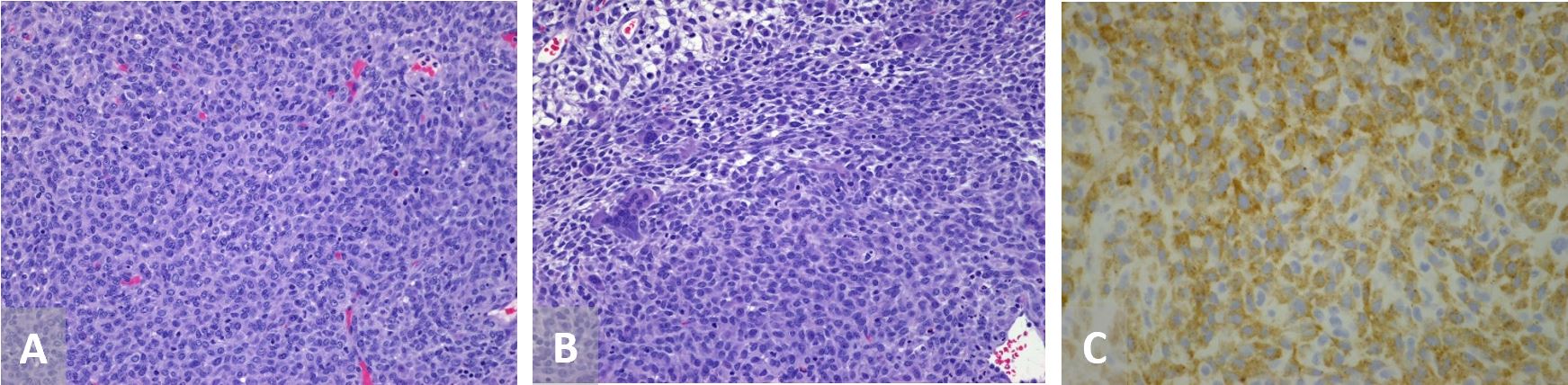
Figure 4. (A) Pathology showing monotonous sheets of cells with ovoid to spindle-shaped nuclei, indistinct pale-gray cytoplasm with a delicate slightly vacuolated chromatin pattern, 200 x. (B) Other features include prominent hemangiopericytomatous vasculature with lymphocytic infiltrate and focal collections of osteoclastic giant cells, 200x. (C) Cells stained for anaplastic lymphoma kinase positivity, 400x.
Treatment
Traditionally, since IMT is noted to be an aggressive and localized disease, treatment options for head and neck IMT have been reported to be managed with complete surgical resction [1,20]. Though, there are cases in which ALK gene positivity can be treated successfully with medical management, such as crizotinib. Crizotinib, a first-generation ALK inhibitor, has been shown to have good response rates in IMT with ALK positivity. Other therapies such as chemotherapy agents and radiotherapy have been described for unresectable tumors [21]. Adjuvant therapies such as steroidal and non-steroidal anti-inflammatory agents have been shown to be beneficial in metastatic disease [1]. In our case, a subtotal to complete glossectomy would have been the surgical treatment. This option would have been extremely morbid in a neonate, so further options were explored including a combination of a surgical debulking of the tongue mass with medical management administration of crizotinib. Crizotinib has possible side effects including visual disorders, gastrointestinal effects, hepatoxicity, pneumonitis, and cardiac effects such as QTc interval prolongation and bradycardia [22].
Outcomes
Outcomes seem to be substantial in surgical interventions, whether complete or partial resections, for treatment of IMTs [5]. Further, when observing the use of an ALK inhibitor agent in ALK positive IMTs, Thielen et al. reported achievement of partial to complete remission in 40% (12/30) patients with use of crizotinib [23]. Another study observed metastatic or inoperable ALK+ IMT reported the overall response rate for patients with IMT was 86% (N=14) with a partial response seen in 36% (5/14) [24]. The recurrence rate has been reported to be low with a 10-year survival rate at approximately 80% [1]. Our unique case showed complete clinical and radiological remission without recurrence at 8 months post-treatment with use of crizotinib.
Inflammatory myofibroblastic tumor is a low-grade malignancy that rarely occurs in the head and neck. To date, there are no reportable cases of IMT with ALK positivity occurring in the tongue in a neonate. Though, surgical resection has been the mainstay of treatment for IMTs, if positive for ALK, crizotinib may be considered. This study shows complete remission in an enlarging tongue mass in a neonate treated with crizotinib with no signs of recurrence at eight months follow-up. Therefore, it is important for clinicians to consider other treatment options for IMT in select patient populations.
Received date: November 25, 2019
Accepted date: February 03, 2020
Published date: February 24, 2020
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2020 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
Supraclavicular flap is an excellent fasciocutaneous flap for head and neck reconstruction due to its close color and texture match. In general, long flaps are required, but with the risk of distal necrosis. The aim of this study is to assess the relationship between the length and distal end necrosis of the supraclavicular flap.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
Authors proposed a simple innovative technique that helps in achieving a clear surgical field at the time of venous repair by diverting the venous bleeding through a glove finger and at the same time preventing venous congestion of the flap.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
The study aims to assess the predictive values of certain psychological factors on the quality of life in patients with Head and Neck Cancer after radiotherapy. The authors conclude that the identification and the understanding of the depressive symptoms of patients, their beliefs about their illness as well as their coping strategies may provide the basis for timely implementation of appropriate intervention that may improve the quality of life in patients.
Biphenotypic sinonasal sarcoma is a rare and aggressive disease that primarily occurs in the sinonasal tract. Diagnosing this type of sarcoma can be challenging due to the need to evaluate both the pathological and immunophenotypic characteristics of the tumor. Furthermore, when it occurs in an unusual location outside the sinonasal tract, it can be confusing for surgeons and result in mismanagement. This article describes a case of biphenotypic sarcoma located in the left parapharyngeal space, which has never been described in English literature before. The authors emphasize the challenges associated with diagnosing and managing this type of tumor.
The Authors report four non-tuberculous granulomatous lymphadenitis cases with temporal and geographic clustering, unresponsive to medical management that warranted modified neck dissection to facilitate cure.
The aim of the study was to characterize children with asymptomatic cervical lymphadenopathy including natural history, radiologic and pathologic findings, and provide guidance in diagnostic and therapeutic intervention and follow-up.
The authors describe their experience in treating severe recurrent respiratory papillomatosis with systemic bevacizumab in two young children with intractable papillomatosis requiring multiple surgical debridements. This is the first report to describe the successful management of recurrent respiratory papillomatosis with systemic bevacizumab in young children. The findings illustrate that systemic bevacizumab can have a dramatic effect on patient outcomes, eliminating the need for repeat surgical interventions. Furthermore, the findings suggest that this novel antiangiogenic agent can safely be used in young children using the same dosing recommendations used in the adolescent and adult populations.
This article presents a case study on the successful replantation of a pediatric lower lip after a dog bite, focusing on artery-only microanastomosis. It highlights the challenges and effective strategies in pediatric microvascular surgery, particularly emphasizing the importance of specialized surgical techniques and thorough postoperative care. Alongside the case study, an extensive literature review supports the feasibility of artery-only anastomosis and the traditional yet critical use of leech therapy for managing venous congestion. This research is vital for medical professionals specializing in pediatric surgery, offering key insights into improving both functional and aesthetic outcomes for young patients. Additionally, it identifies gaps in long-term research and stresses the need for ongoing studies to refine treatment protocols, making it an indispensable resource for enhancing patient care and outcomes in pediatric reconstructive surgeries.
This investigation delineates a pivotal association between socioeconomic inequities, quantified via the Area Deprivation Index (ADI), and an elevated incidence of button battery ingestion in pediatric populations, highlighting a profound public health issue. The results indicate that children residing in socioeconomically disadvantaged areas are at an increased risk of sustaining severe injuries from the ingestion of button batteries, which could lead to elevated morbidity and mortality rates. The study urgently calls for immediate diagnostic and therapeutic interventions to avert critical health complications and delineates the complex pathophysiology underlying button battery injuries. For clinicians and healthcare practitioners, particularly those within pediatrics and emergency medicine, this manuscript is indispensable. It provides deep insights into the ramifications of socioeconomic disparities on health outcomes, fosters the refinement of diagnostic and therapeutic modalities, and champions preventive initiatives. The authors advocate for intensified parental awareness, the redesign of battery products to enhance safety, and the formulation of healthcare policies that promote equity, aiming to curtail this escalating health challenge.
This is a well-written case report. The abstract provides essential information which allows for easier retrieval from electronic database. The introduction is concise. The details of the clinical presentation and examinations are provided, including those from imaging and laboratory studies. I believe this article is suitable for publication in its current form.
ResponseWe appreciate the feedback. Thank you for reviewing our article.
The essential features of the case report are properly summarized in the Conclusion section. The experiences that may be learnt from the case report is well stated. It would be better if the conclusion could be more concise and shorter.I wonder if the patient had electrocardiograms and cardiology follow-ups given asymptomatic QT prolongation is a known side effect of crizotinib.
ResponseThe conclusion was edited to read in a more concise manner.The patient did have QTc prolongation and she was seen by Cardiology. She underwent a baseline ECHO and monitored with serial EKGs. Possible risks/side effects of the medication was added to this article along with noting she had a prolonged QTc interval.
Baumanis MM, Nicklaus P, Gener M. ALK+ inflammatory myofibroblastic tumor of the tongue in a neonate treated with crizotinib. Arch Otorhinolaryngol Head Neck Surg 2020;4(1):3. https://doi.org/10.24983/scitemed.aohns.2020.00124