Biphenotypic sinonasal sarcoma is a rare, locally aggressive disease. This is a site-specific sarcoma that commonly develops in the sinonasal tract. Identifying biphenotypic sinonasal sarcoma is difficult due to the need to assess both the pathological and immunophenotypic characteristics of the tumor. When an unusual presentation occurs at a different site altogether, it may be confusing for the operating surgeon, leading to mismanagement of the patient's condition. We present a rare and unique case of biphenotypic sarcoma of the left parapharyngeal space that has not been previously reported in the English literature because of its site specificity. Occasionally, a biphenotypic sarcoma can develop outside the nasal cavity and paranasal sinuses. Diagnosis and management of this condition are both challenging for pathologists and surgeons.
Biphenotypic sinonasal sarcoma is a newly described tumor of the sinonasal region, as defined in the most recent edition of the World Health Organization's head and neck tumors [1]. Despite its unique dual staining patterns for neural and myogenic markers, it can show some histological overlap with other tumors, such as fibrosarcomas, monophasic synovial sarcomas, peripheral nerve sheath tumors, glomangiopericytomas, and solitary fibrous tumors [2]. In view of the indolent nature of biphenotypic sinonasal sarcoma, it is imperative to determine its exact diagnosis. Immunohistochemistry as well as molecular confirmatory testing would be required to make the diagnosis, predict a course of treatment, and avoid overtreatment or undertreatment. The PAX3-MAML3 fusion is the most common genetic alteration in this tumor (58.6%), but isolated PAX3 rearrangements (19.2%), absence of rearrangements (9.1%), PAX3-FOXO1 (8.1%), PAX3-NCOA1 (4%), and isolated MAML3 rearrangements (2%) have also been reported [3]. Aside from the dual staining pattern, it is also characterized by a highly cellular spindle cell neoplasm with monomorphic histopathology, as well as S-100 positivity and actin positivity on immunophenotyping.
The true nature and full information regarding biphenotypic sinonasal sarcoma are still unknown. This is because it is relatively rare and has been reported mainly in case reports and small case series. Biphenotypic sarcomas have been reported in the English literature at various sites of the nose and paranasal sinuses with intracranial or orbital extensions. This case report discusses the diagnosis and management of the first ectopic site of these tumors located in the parapharyngeal space.
A 51-year-old male presented to the outpatient department complaining of diffuse pain on his left side of the neck for the past six years. There was a dull aching pain that was insidious in onset, non-progressive, intermittent, and sometimes radiating to the left forearm. Neither tingling nor numbness was present in the forearm, nor were swallowing difficulties or voice changes reported. The condition was not aggravated or relieved by any factors. In the past six years, the patient had been taking continuous medication (Metformin and Glimepiride) for type 2 diabetes mellitus. Additionally, the patient was hypertensive and had been on continuous medication for the past 10 months (Amlodipine and Telmisartan). A general physical examination revealed no abnormalities, and vital signs were within normal limits.
During the local examination, no swelling was apparent, but upon palpation, a diffuse swelling was palpable over the left side of the neck, which was non-tender, firm to solid in consistency, non-mobile, and had diffuse margins. The overlying temperature was normal. An indirect laryngoscopy using a 70-degree Hopkins rigid endoscope revealed no abnormalities.
The patient underwent radiological investigations including contrast enhanced magnetic resonance imaging cervical spine with screening of whole spine. The results revealed a large lobulated heterogeneous signal intensity mass lesion on the left side of the neck. This mass primarily affected the post-styloid parapharyngeal and prevertebral spaces and displaced the internal jugular vein and carotid artery anteriorly against the left anterolateral vertebral margins of the C2 to C6 vertebrae. There was evidence of muscle involvement in the short-tau inversion-recovery (STIR) sequences, particularly the longus colli, the prevertebral muscles, and the scalene group of muscles with heterogeneous hyperintensities. The mass appeared mildly hyperintense on T2 images with peripheral hypointensities. The tumor was isointense on T1 images with focal areas of peripheral hyperintensity, and heterogeneously hyperintense on STIR images, measuring approximately 6 cm x 3.9 cm x 12.8 cm in size. Although the internal jugular vein was compressed, the flow voids in the vascular system remained largely intact. The neck did not exhibit any significant lymph nodes, but subcentimetric lymph nodes with fatty hilum were present bilaterally at levels II and Ib of the neck. The mass indented the laryngopharynx and contralaterally displaced it right laterally, but no obvious invasion was apparent (Figure 1).
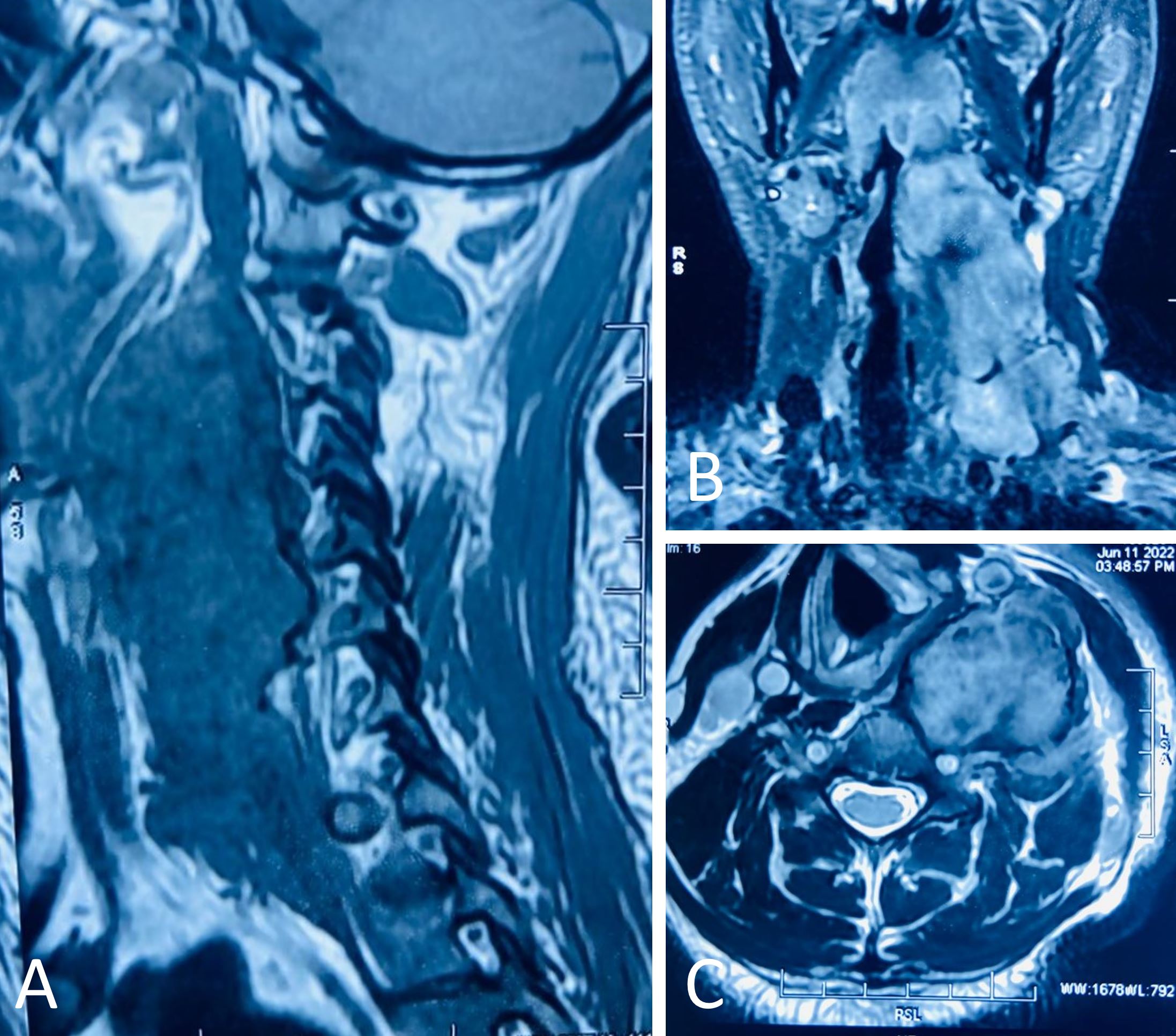
Figure 1. A contrast enhanced magnetic resonance image of the patient's neck reveals a heterogeneous lobulated mass measuring 6 cm x 3.9 cm x 12.8 cm within the perivertebral and post-styloid parapharyngeal regions. (A) A sagittal section of C2 to C6 reveals abutting anterolateral margins (underlying marrow edema and variable cortical flattening in C2 to C5 vertebral bodies, and likely mass extension in C2 to C3). In the perivertebral muscles and the scalene muscles (likely involved), there is a heterogeneous hyperintensity of the short-tau inversion-recovery (STIR) sequences. Coronal (B) and axial (C) sections show the mass in the post-styloid parapharyngeal region and perivertebral areas, displacing the laryngopharynx towards the right without any apparent invasion.
Ultrasound-guided fine needle aspiration revealed a spindle cell lesion of possible neural origin with borderline cytological features that would require histopathological verification. An orthopedic spine surgeon and a plastic surgeon were consulted in order to plan the surgery and provide assistance during the procedure.
As shown in Figure 2A, the patient was then scheduled for excision of the mass under general anesthesia. As the majority of the tumor's bulk lay medial to the sternocleidomastoid and omohyoid muscles, these muscles were cut in the middle in order to expose the tumor (Figure 2B). Upon dissection, the tumor appeared to be bilobed in appearance, extending superiorly just medial to the angle of the mandible and inferiorly until the apex of the pleura. The spinal accessory nerve and the vagus nerve were identified and preserved. The intraoperative examination revealed a few enlarged lymph nodes. A small tumor tissue with two lymph nodes was sent for frozen sectioning intraoperatively, which revealed spindle cells with many nuclei. There was no evidence of mitosis or necrosis. In this case, the tissue had neural origins, suggesting that it may have been the result of a neural tumor. However, a section taken from the lymph node demonstrated reactive lymphoid hyperplasia. Following dissection, we found that the tumor was firmly adherent to the body of cervical vertebrae C3-C6 and had extended into the transverse processes of C-3, C4, and C5. By using a chisel and hammer, the tumor was separated from the vertebral bodies from anterior to posterior (Figure 2C). As the tumor extended below the clavicle, the thoracic duct was inadvertently injured during dissection in that area. The specimen was removed in total (Figure 2D).
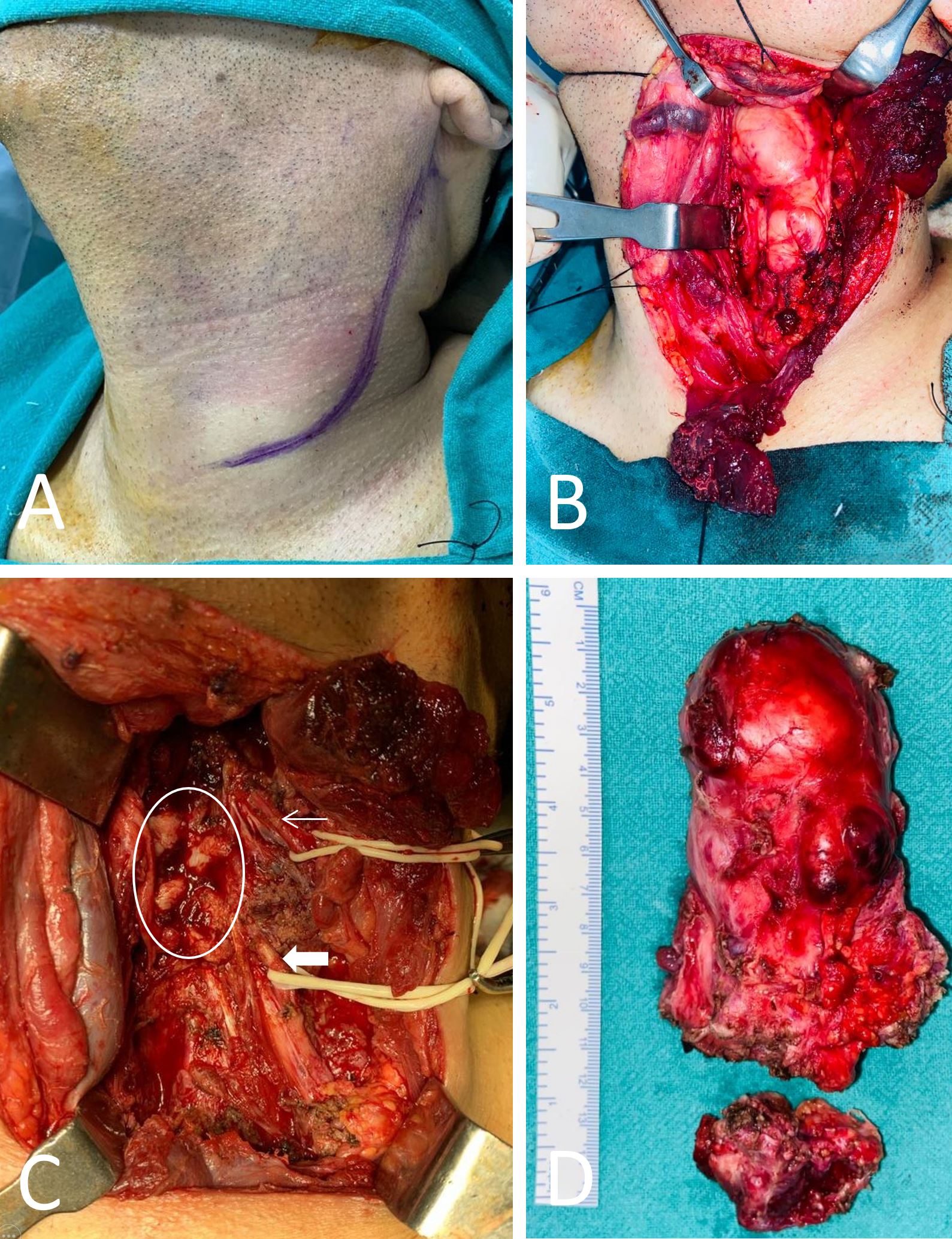
Figure 2. Photographs taken before and during surgery. (A) There is a J-shaped incision on the left side of the neck, and there is no apparent swelling visible on the neck. (B) Upon dividing the sternocleidomastoid muscle, the mass is exposed. (C) An intraoperative photograph following the excision of the tumor. The thin arrow indicates a spinal accessory nerve. The thick arrow represents the vagus nerve. The circle indicates the transverse processes of the cervical vertebrae. (D) Upon excision of the tumor, a specimen is obtained.
The wound was closed in layers with a Hemovac drain. The patient was transferred to the Intensive Care Unit for overnight observation and extubated the following day. It was a pleasant surprise to find that there were no neurological deficits postoperatively, and the patient was discharged on postoperative day five. Serial sectioning of the specimen revealed a grey, white growth measuring 9x4x3 cm3 roughly reaching up to all margins. Microscopically, the growth was composed of spindle cells. It was observed that these cells were monotonous in appearance with an ovoid to elongated nucleus. Pleomorphism was minimal, and there was no mitosis. The tumor had infiltrated adjacent tissues and skeletal muscles, entangling the blood vessels. Hemorrhages were present in certain areas. Immunohistochemistry reveals the expression of Vimentin, Desmin, smooth muscle actin, and S100 in tumor cells. There was no evidence of CD34 or CK expression in tumor cells (Figure 3). There was a strong indication that the tumor was a biphenotypic sinonasal sarcoma based on these features. To determine the condition of the paranasal sinuses and whether a coexisting sinonasal mass was present, the patient was advised to undergo non-contrast computed tomography of the nose and paranasal sinuses. The report revealed that there were no sinonasal masses (Figure 4). Upon being referred to the radiation oncology department, the patient was scheduled for concurrent radiotherapy.
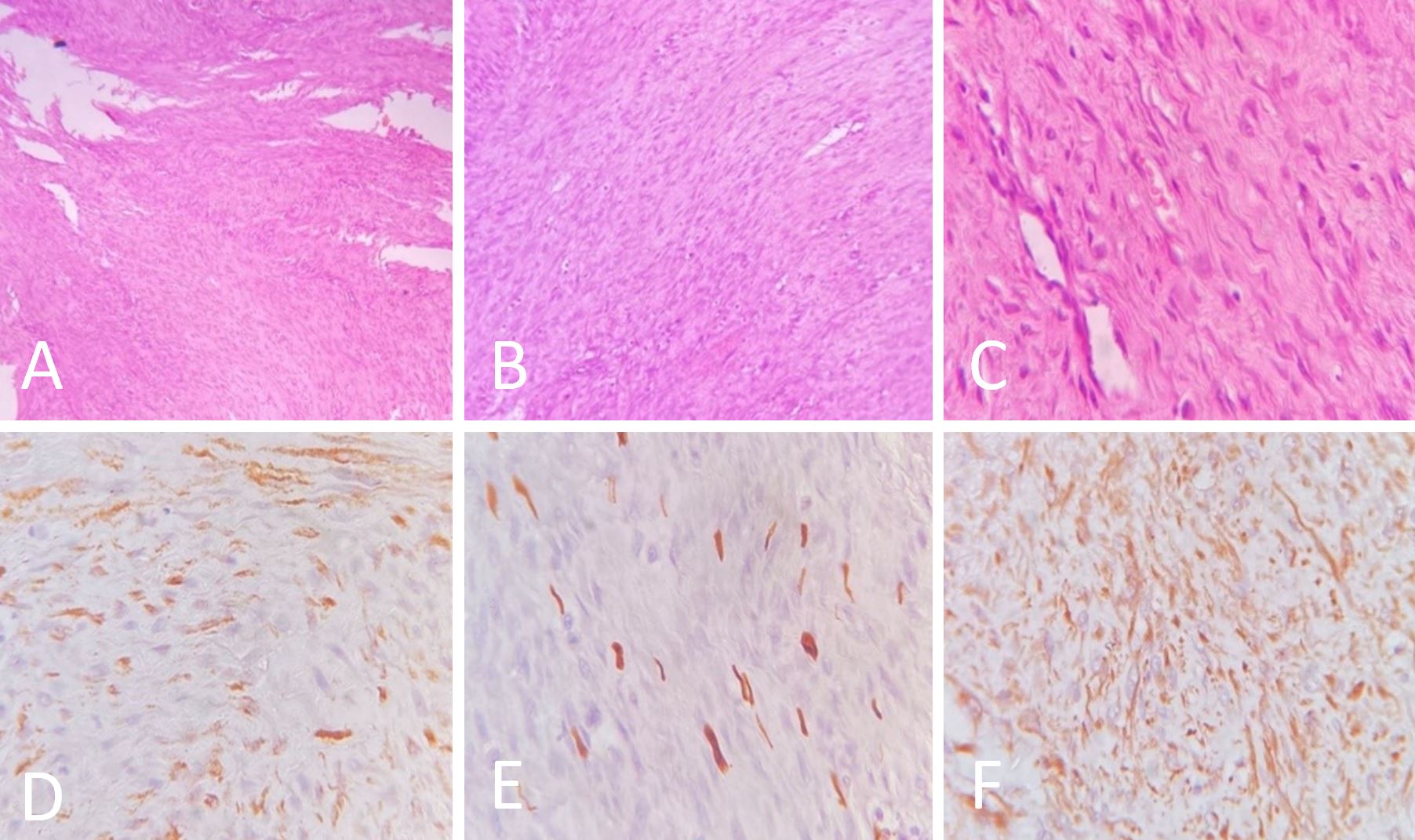
Figure 3. Hematoxylin and eosin-stained sections of the specimen are shown in the image. (A) Monomorphic spindle cells with ovoid to elongated nuclei (40X). (B) Spindle cells with inconspicuous nucleoli (100X). (C) Minimal pleomorphism (400X). (D) Diffuse cytoplasmic and membranous staining with smooth muscle actin (400X). (E) Focal nuclear and cytoplasmic positivity with S100 (400X). (F) Diffuse cytoplasmic positivity with Vimentin (400X).
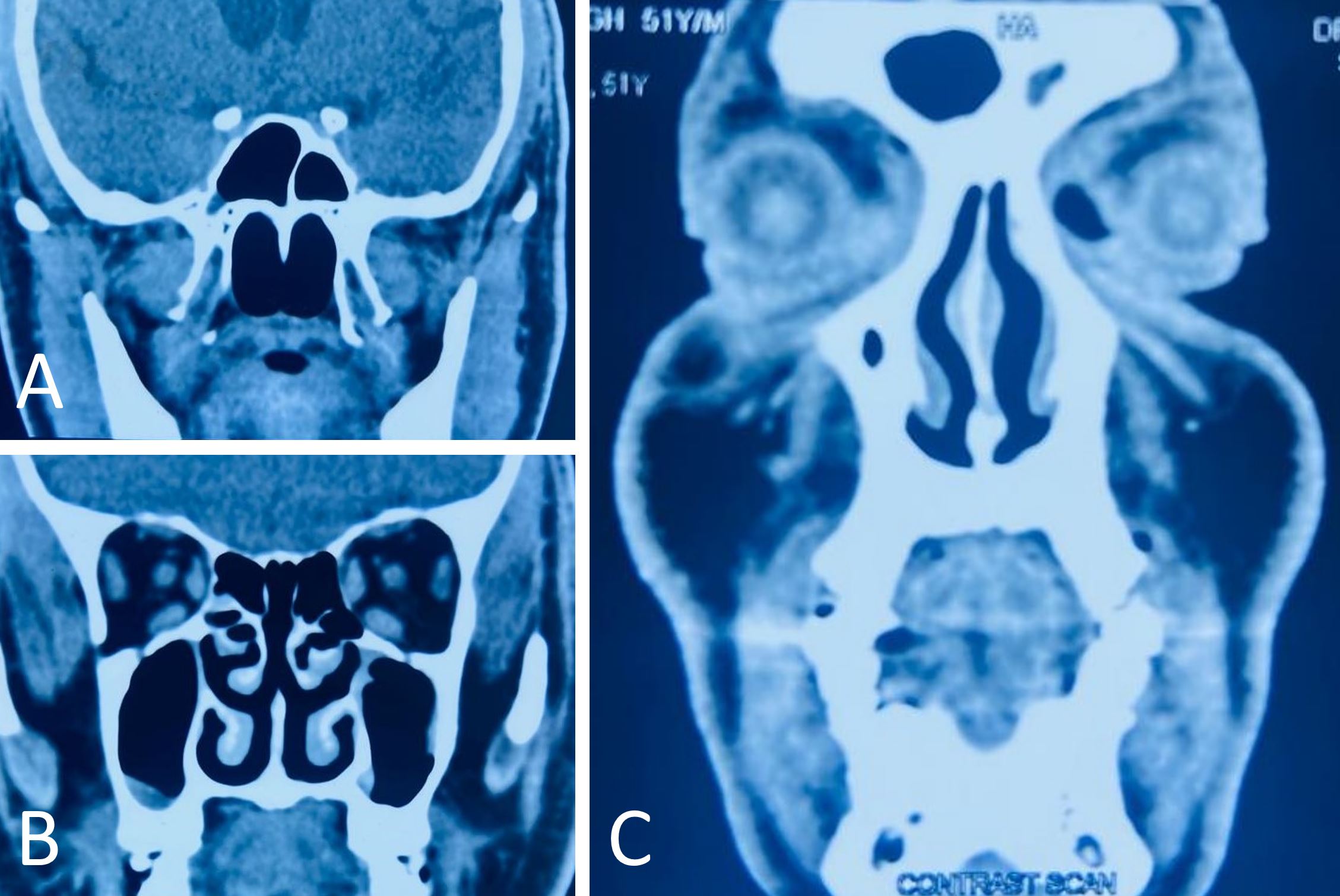
Figure 4. A non-contrast computed tomography examination of the nose and paranasal sinuses reveals that no masses are found in the sinonasal cavities (A-C).
Initially described by Lewis et al. in 2012, biphenotypic sinonasal sarcoma was described as a low-grade sinonasal sarcoma with neural and myogenic features [4]. Furthermore, they observed histological similarity between this group of cases and adult fibrosarcoma, monophasic synovial sarcoma, and malignant peripheral nerve sheath tumor (MPNST). The tumor was renamed biphenotypic sinonasal sarcoma in 2014 due to its recurrent genetic rearrangement in PAX3 [5]. It is imperative to note that the tumor is locally invasive and may affect adjacent areas including the skull base frontal lobe and the opposite side sinuses if diagnosis or treatment is delayed [6]. We also found that the tumor infiltrated the prevertebral fascia in our case. It has a female to male ratio of 2:1, indicating a preference for women, whereas our case report refers to a male patient. It is typically associated with multiple sinonasal subsites, with the superior nasal cavity and ethmoid sinus being the most commonly affected, followed by the sphenoid sinus [7]. In a case of biphenotypic sinonasal sarcoma of the nose and paranasal sinuses, the most common symptoms are nasal obstruction and midfacial pressure; however, patients may also experience epistaxis, epiphora, rhinorrhea, and recurrent sinusitis [3]. However, none of these symptoms were present in our case. Frichie et al. reported the largest series of biphenotypic sinonasal sarcomas, including 44 patients, all of whom had a disease affecting either their nose or their paranasal sinuses [8]. In a series of 41 cases reported by Loarer et al. in 2019, all of the cases originated from the nose and paranasal sinuses, and none of them presented as ectopic [9]. Clinically, a biphenotypic sinonasal sarcoma may resemble a schwannoma in both appearance and presentation. In our case, ultrasonography-guided fine needle aspiration cytology revealed a spindle cell lesion with borderline cytological characteristics.
The surgical excision of the case was planned accordingly. The intraoperative appearance of the case was similar to that of a schwannoma and appeared to arise from the brachial roots. However, the surgical management of such tumors may require the cooperation of a multidisciplinary team since the tumor was located near the vertebrae and brachial roots. In order to reduce the risk of neurovascular injuries during surgery, otolaryngologists, orthopedic spine surgeons, and plastic surgeons should be present during surgery. To accurately diagnose biphenotypic sinonasal sarcoma, immunophenotyping and/or molecular analysis are required. In histological terms, biphenotypic sinonasal sarcoma is characterized by highly cellular spindle cell neoplasm with monomorphic features on histology as well as immunophenotyping positive for both S-100 and actin.
Biphenotypic sinonasal sarcomas are generally reported to originate from the nasal cavity, sinuses, and adjacent tissues; however, this was not the case in our case. An unusual presentation was observed in our patient. He complained of diffuse left-sided neck pain not previously described in the English literature. A radiographic examination revealed that the tumor was not involving its usual sites, namely the nose and paranasal sinuses. It was noted that the tumor mass was located in the left parapharyngeal space and that it involved the pre- and perivertebral spaces. A cytological examination revealed spindle cells with neural origins. It appeared that this tumor had infiltrated the surrounding tissues and skeletal muscles, entrapping blood vessels along the way. In immunohistochemistry, tumor cells were found to express Vimentin (a marker of mesenchymal tissue), S-100 (a marker of neural tissue), as well as Desmin and smooth muscle actin (representing muscular origins) [10,11]. There is a tendency for biphenotypic sinonasal sarcomas to recur, and the recurrence rates between surgical excision alone and surgical excision with radiotherapy are equivalent. Indications for postoperative radiotherapy include positive or close surgical margins, high-grade tumors, perineural invasion, or concerns regarding surgical margins. Typically, radiotherapy is used to target the tumor bed, resection cavity, and areas that are at high risk of harboring microscopic disease [12].
It is pertinent to note a few salient points. First, biphenotypic tumors can be challenging to diagnose both for clinicians and pathologists. Therefore, it is imperative that the specimen be reviewed by an experienced pathologist in order to plan appropriate surgical management. Second, biphenotypic tumors should be considered among the differential diagnoses for neck swelling. Third, such tumors should be managed by a multidisciplinary team.
Biphenotypic sinonasal sarcomas are rare sinonasal tumors that are generally not found in ectopic sites such as the parapharyngeal and paravertebral space. To make an accurate diagnosis and to manage the case effectively, cytology and histopathology must be thoroughly examined by an experienced pathologist.
Received date: August 11, 2022
Accepted date: November 02, 2022
Published date: February 20, 2023
The manuscript has not been presented at any meetings on the topic.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The authors obtained permission from the participants in the human research prior to publishing their images or photographs.
This research has received no specific grant from any funding agency either in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest declared by either the authors or the contributors of this article, which is their intellectual property.
It should be noted that the opinions and statements expressed in this article are those of the respective author(s) and are not to be regarded as factual statements. These opinions and statements may not represent the views of their affiliated organizations, the publishing house, the editors, or any other reviewers since these are the sole opinion and statement of the author(s). The publisher does not guarantee or endorse any of the statements that are made by the manufacturer of any product discussed in this article, or any statements that are made by the author(s) in relation to the mentioned product.
© 2023 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). In accordance with accepted academic practice, anyone may use, distribute, or reproduce this material, so long as the original author(s), the copyright holder(s), and the original publication of this journal are credited, and this publication is cited as the original. To the extent permitted by these terms and conditions of license, this material may not be compiled, distributed, or reproduced in any manner that is inconsistent with those terms and conditions.
Supraclavicular flap is an excellent fasciocutaneous flap for head and neck reconstruction due to its close color and texture match. In general, long flaps are required, but with the risk of distal necrosis. The aim of this study is to assess the relationship between the length and distal end necrosis of the supraclavicular flap.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
Authors proposed a simple innovative technique that helps in achieving a clear surgical field at the time of venous repair by diverting the venous bleeding through a glove finger and at the same time preventing venous congestion of the flap.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
The authors present a case of a neonate born with an enlarging tongue mass whose biopsy revealed IMT with ALK positivity. Treatment included tumor debulking and an ALK inhibitor, crizotinib, which resulted in complete remission.
The study aims to assess the predictive values of certain psychological factors on the quality of life in patients with Head and Neck Cancer after radiotherapy. The authors conclude that the identification and the understanding of the depressive symptoms of patients, their beliefs about their illness as well as their coping strategies may provide the basis for timely implementation of appropriate intervention that may improve the quality of life in patients.
Esophageal granular cell tumors (GCT) represent a rare entity of tumors of the esophagus. Patients with esophageal GCTs are usually asymptomatic, with the lesion most commonly presenting as an incidental finding on endoscopy. The GCTs of the esophagus are poorly understood in medical literature. It is unknown if they undergo malignant degeneration, whether the malignancy can be diagnosed preoperatively, and how the tumor can be managed. The authors evaluated the clinical and pathologic features of all esophageal GCTs at their institution to understand them better.
This study reviews the reconstruction modalities within the multidisciplinary approach for chest wall reconstruction. The authors have proposed an algorithm that is formulated based on their experience to manage chest wall defects following cancer resection.
The authors present a case of a neonate born with an enlarging tongue mass whose biopsy revealed IMT with ALK positivity. Treatment included tumor debulking and an ALK inhibitor, crizotinib, which resulted in complete remission.
The incidence of high-grade sinonasal adenocarcinomas of non-intestinal origin is extremely rare. In this case report, the authors present a very rare case of high-grade non-intestinal sinonasal adenocarcinoma presenting with a challenging diagnosis. Because of its significantly different prognosis, this study provides a detailed explanation of how the authors differentiate it from other sinonasal tumors. In addition, they describe how a radical endoscopic resection was applied in order to achieve a total excision.
The article discusses a complex case of a 51-year-old Chinese woman diagnosed with a pituitary neuroendocrine tumor in the clivus, characterized by its invasive nature and atypical symptoms, leading to diagnostic challenges between chordoma and chondrosarcoma. Achieving a correct diagnosis through a transsphenoidal biopsy enabled effective surgical removal of the tumor without complications. Highlighting the critical role of biopsy for accurate diagnosis, especially with atypical imaging, the study showcases the efficacy of minimally invasive transnasal endoscopic biopsy techniques. It emphasizes the importance of a multidisciplinary approach for optimal patient outcomes in complex pituitary tumors, underlining the need for vigilance and adaptability in managing such rare conditions. This contributes valuable insights to the medical field, particularly for neurosurgery, otorhinolaryngology, and endocrinology practitioners.
This manuscript showcases an advanced surgical approach for treating malignant giant cell tumor of bone, emphasizing precision and ethical considerations. It leverages innovative pedicled flap technologies, as opposed to free flaps, enhancing limb functionality and patient quality of life. This technique equips surgeons with evidence that tailored surgical strategies can significantly improve outcomes in complex cases. The paper discusses technical challenges and highlights the application of supercharging and superdrainage techniques in limb reconstructions, methods well-established in microsurgery but infrequently used in oncological contexts. These techniques are crucial for optimizing flap viability and ensuring surgical success. Additionally, the manuscript underscores the profound impact of these advancements on patient lives, offering hope and showcasing tangible benefits. This narrative, blending scientific analysis with patient stories, enriches the understanding of limb reconstruction innovations in oncological surgery, making it invaluable for surgeons.
Singh I, Sharma R, Negi S, Gopal A, Mallya V, Arora S, Bhandari PS. Biphenotypic spindle cell sarcoma: First report of an ectopic occurrence in the parapharyngeal space. Arch Otorhinolaryngol Head Neck Surg 2023;7(1):1. https://doi.org/10.24983/scitemed.aohns.2023.00169